- Record: found
- Abstract: found
- Article: found
Informed Consent in Biomedical Research
Read this article at
Abstract
Informed consent is the result of tumultuous events in both the clinical and research arenas over the last 100 years. Throughout this time, the notion of informed consent has shifted tremendously, both due to advances in medicine, as well as the type of data being gathered. As such, informed consent has misaligned with the goals of medical research. It is becoming more and more vital to address this chasm, and begin building new frameworks to link this disconnect. Thus, we address three goals in this paper. First, we discuss the history of informed consent and unify the varying definitions of the term. Second, we evaluate the current research on the topic, classify them into themes, and attend to the problems therein. Lastly, we employ these themes of informed consent research mentioned previously to provide guidance and insight for future research in the arena.
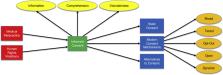
Graphical Abstract
Related collections
Most cited references64
- Record: found
- Abstract: found
- Article: not found
Identifying personal genomes by surname inference.

- Record: found
- Abstract: found
- Article: found
Improving understanding in the research informed consent process: a systematic review of 54 interventions tested in randomized control trials
- Record: found
- Abstract: found
- Article: not found
