- Record: found
- Abstract: found
- Article: found
Dopamine and Somatostatin Analogues Resistance of Pituitary Tumors: Focus on Cytoskeleton Involvement
Read this article at
Abstract
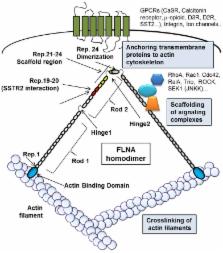
Pituitary tumors, that origin from excessive proliferation of a specific subtype of pituitary cell, are mostly benign tumors, but may cause significant morbidity in affected patients, including visual and neurologic manifestations from mass-effect, or endocrine syndromes caused by hormone hypersecretion. Dopamine (DA) receptor DRD2 and somatostatin (SS) receptors (SSTRs) represent the main targets of pharmacological treatment of pituitary tumors since they mediate inhibitory effects on both hormone secretion and cell proliferation, and their expression is retained by most of these tumors. Although long-acting DA and SS analogs are currently used in the treatment of prolactin (PRL)- and growth hormone (GH)-secreting pituitary tumors, respectively, clinical practice indicates a great variability in the frequency and entity of favorable responses. The molecular basis of the pharmacological resistance are still poorly understood, and several potential molecular mechanisms have been proposed, including defective expression or genetic alterations of DRD2 and SSTRs, or an impaired signal transduction. Recently, a role for cytoskeleton protein filamin A (FLNA) in DRD2 and SSTRs receptors expression and signaling in PRL- and GH-secreting tumors, respectively, has been demonstrated, first revealing a link between FLNA expression and responsiveness of pituitary tumors to pharmacological therapy. This review provides an overview of the known molecular events involved in SS and DA resistance, focusing on the role played by FLNA.
Related collections
Most cited references92
- Record: found
- Abstract: found
- Article: not found
Filamins as integrators of cell mechanics and signalling.
- Record: found
- Abstract: found
- Article: not found