- Record: found
- Abstract: found
- Article: found
Expression of GLP-1 receptor and CD26 in human thyroid C-cells: The association of thyroid C-cell tumorigenesis with incretin-based medicine
Read this article at
Abstract
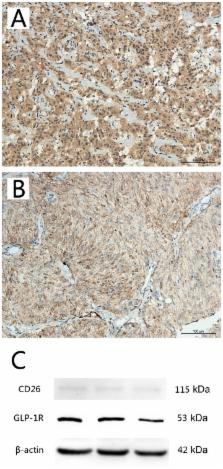
Recent reports have demonstrated that long-term and high dosage treatments with incretin-based medicine, such as hormone glucagon-like peptide-1 (GLP-1) may induce thyroid C-cell pathological changes in rodents, rather than in humans. Doubts regarding the tumorigenic potential of GLP-1 analogues in human thyroid C-cells remain. The present study aimed to determine the expression levels of GLP-1 receptor (GLP-1R) and cluster of differentiation 26 (CD26) in the C-cells of thyroid tissues from non-neoplastic, medullary carcinoma and hyperplasia subjects, and to explore the potential clinical significance. The following cases were analyzed: Medullary thyroid carcinoma (n=62, including 59 paraffin-embedded samples and 3 fresh frozen samples), C-cell hyperplasia (n=20, paraffin-embedded samples) and non-neoplastic thyroid tissue samples (n=7, paraffin-embedded samples). GLP-1R and CD26 expression was detected using immunohistochemical staining and western blotting. There were significant differences in the expression levels of the two markers between medullary thyroid carcinoma and C-cell hyperplasia, in addition to between medullary thyroid carcinoma and non-neoplastic thyroid tissue following immunohistochemical staining. Similar significant differences in the expression of GLP-1R and CD26 were detected using western blot analysis in the medullary thyroid carcinoma compared with non-neoplastic thyroid tissue sectioned from the aforementioned fresh frozen samples. There was a significant negative correlation between GLP-1R and CD26 expression. In addition, the present data indicated that GLP-1R expression was associated with the age of the patients with medullary thyroid carcinoma. These results suggested that GLP-1R and CD26 may be closely associated with the development of thyroid C-cell hyperplasia and medullary thyroid carcinoma, and indicated the importance of being aware of the side effects of incretin medicine.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Dipeptidyl-peptidase IV (CD26)--role in the inactivation of regulatory peptides.
- Record: found
- Abstract: found
- Article: not found
Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation.
- Record: found
- Abstract: found
- Article: not found