- Record: found
- Abstract: found
- Article: found
Prostate cancer addiction to oxidative stress defines sensitivity to anti-tumor neutrophils
Read this article at
Abstract
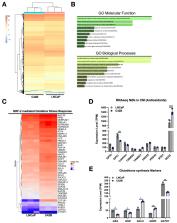
Bone metastatic prostate cancer (BM-PCa) remains one of the most difficult cancers to treat due to the complex interactions of cancer and stromal cells. We previously showed that bone marrow neutrophils elicit an anti-tumor immune response against BM-PCa. Further, we demonstrated that BM-PCa induces neutrophil oxidative burst, which has previously been identified to promote primary tumor growth of other cancers, and a goal of this study was to define the importance of neutrophil oxidative burst in BM-PCa. To do this, we first examined the impact of depletion of reactive oxygen species (ROS), via systemic deletion of the main source of ROS in phagocytes, NADPH oxidase (Nox)2, which we found to suppress prostate tumor growth in bone. Further, using pharmacologic ROS inhibitors and Nox2-null neutrophils, we found that ROS depletion specifically suppresses growth of androgen-insensitive prostate cancer cells. Upon closer examination using bulk RNA sequencing analysis, we identified that metastatic prostate cancer induces neutrophil transcriptomic changes that activates pathways associated with response to oxidative stress. In tandem, prostate cancer cells resist neutrophil anti-tumor response via extracellular (i.e., regulation of neutrophils) and intracellular alterations of glutathione synthesis, the most potent cellular antioxidant. These findings demonstrate that BM-PCa thrive under oxidative stress conditions and such that regulation of ROS and glutathione programming could be leveraged for targeting of BM-PCa progression.
Related collections
Most cited references75
- Record: found
- Abstract: found
- Article: not found
STAR: ultrafast universal RNA-seq aligner.
- Record: found
- Abstract: found
- Article: not found