- Record: found
- Abstract: found
- Article: found
Reining in the CD8+ T cell: Respiratory virus infection and PD-1-mediated T-cell impairment
review-article
3 January 2019
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Introduction
Viral acute respiratory infections (ARIs) are associated with cluster of differentiation
(CD)8+ T cells that exhibit diminished production of cytokines and cytotoxic molecules.
Though these cells recognize major histocompatibility complex (MHC)-I–restricted viral
epitopes, ex vivo stimulation of these cells with these same viral peptides fails
to elicit production of interferon (IFN)γ, interleukin (IL)-2, or tumor necrosis factor
(TNF)-α; degranulation as measured by CD107a staining; or other features of functional
CD8+ T cells. This is a unique feature of viral ARI because T-cell dysfunction occurs
in chronic rather than acute infections of many other organs. Although CD4+ regulatory
T cells (Tregs) contribute to CD8+ T-cell dysfunction, recent work on a variety of
viruses has identified inhibitory receptors as a key mediator of this phenotype. Programmed
cell death 1 (PD-1) is the most well-studied inhibitory receptor in T-cell impairment,
but there is growing evidence that other inhibitory receptors also play a role. The
tendency of CD8+ T cells to have significantly reduced functionality in the context
of respiratory virus infection has been termed "T cell impairment".
What is T-cell impairment?
T-cell immunity, especially CD8+ T cells, is essential to clearing acute viral lung
infections. In mouse models, absence of CD8+ T cells leads to delayed clearance of
viruses, whereas humans that have defects in T-cell immunity associated with aging,
immune suppression, or cancer tend to have more severe infections and poorer outcomes
[1, 2]. However, despite this clear need for CD8+ T-cell–mediated immunity, infections
due to a broad range of acute viruses, including influenza virus, respiratory syncytial
virus (RSV), pneumonia virus of mice, vaccinia virus (respiratory but not systemic
infection), human metapneumovirus (HMPV), and others, have been associated with a
state called T-cell impairment [3–11]. Broadly defined, T-cell impairment occurs when
virus-specific CD8+ T cells fail to produce inflammatory cytokines or perform cytotoxic
functions at the site of infection, while the same virus-specific cells at other sites,
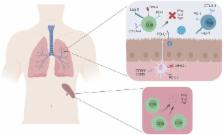
such as the spleen, are completely functional (Fig 1). Epitope-specific cells in the
lung-draining mediastinal lymph nodes are completely functional [7]. This suggests
that the inflammatory environment of the lung parenchyma drives the process of T-cell
impairment during ARI.
10.1371/journal.ppat.1007387.g001
Fig 1
Respiratory virus infection is associated with T-cell impairment.
During respiratory virus infection, impaired virus-specific T cells in the lung fail
to produce pro-inflammatory cytokines or perform cytotoxic functions. T-cell impairment
is at least partially mediated by expression of inhibitory receptors, which decrease
effector T-cell function and promote Treg activity. In contrast, virus-specific T
cells in the spleen rarely express inhibitory receptors and are functional. PD-1 and
other inhibitory receptors are expressed in the lung during both primary infection
and reinfection. CD8, cluster of differentiation 8; CTLA-4, cytotoxic T-lymphocyte-associated
protein 4; IFNƔ, interferon gamma; IL-2, interleukin 2; Lag-3, Lymphocyte-activation
gene 3; MHCII, major histocompatibility complex class II; PD-1, programmed cell death
1; PD-L1,; Tim-3, T-cell immunoglobulin and mucin-domain containing-3; TNF, tumor
necrosis factor; Treg, regulatory T cells.
T-cell impairment requires cognate antigen, because CD8+ T cells that are not specific
to a viral antigen remain functional during primary infection [4]. It also appears
to require active infection, because dendritic cell vaccination leads to functional
virus-specific CD8+ T cells [4]. However, adoptive transfer of cells into a naïve
lung causes the transferred cells to lose some effector potential, though infection
further decreases the function of these cells, indicating that the lung environment
itself may program some degree of suppression even in settings without infection [12,
13]. Impairment also occurs during secondary infections [3–7, 14], which may indicate
one reason why humans have poor T-cell memory to respiratory viruses and are susceptible
to reinfection.
Why would the lung favor T-cell impairment?
As the site of gas exchange, the lung is essential for the long-term survival of an
organism. Left unchecked, effector T cells, inflammatory cytokines, and other immune
mediators could damage healthy tissue alongside virus-infected cells. Indeed, a significant
portion of lung injury after serious infections is due to immunopathology rather than
to the virus infecting and killing cells [15, 16]. Therefore, it is necessary for
the immune system to balance virus clearance and immune-mediated damage during infection,
and CD8+ T-cell impairment likely represents a host mechanism of protective immunoregulation.
Similarly, once a respiratory virus is cleared, normal lung homeostasis must be restored.
T-cell impairment likely represents a regulatory mechanism to restore the normal state
by reducing function or survival of cytotoxic virus-specific CD8+ T cells. Some strategies
to reverse T-cell impairment, discussed below, can be associated with increased immunopathology
or disease severity and delayed recovery from weight loss [4, 17, 18] at time points
after viral inoculation when the virus has almost entirely been cleared.
Another tissue that appears to favor T-cell impairment is the brain. Some neurotropic
infections, such as the JHM strain of murine coronavirus, cause dysfunctional virus-specific
T cells [19]. Considering the critical and delicate nature of neural tissue and the
limited healing capacity of the brain, it seems likely that T-cell impairment in both
organs represents an adaptation to preserve vital organs during infection and recovery.
Reversal of T-cell dysfunction in neurotropic infection also causes increased immunopathology
and disease severity [19].
What mediates T-cell impairment?
Much work remains to fully elucidate the mechanisms of impairment. One essential component
is the action of inhibitory receptors, the most well-described being PD-1. PD-1 and
other inhibitory receptors interfere with signaling through the T-cell receptor and
thus have far reaching consequences on T-cell function, from cytokine release to metabolism
[20–22]. PD-1 signaling alters metabolism in multiple ways: it suppresses PI3k (phosphatidylinositide
3-kinase/Akt and mTOR (mammalian target of rapamycin) activation, diverts metabolic
pathways away from glycolysis and towards fatty acid oxidation, and increases reactive
oxygen species that promote apoptosis [21, 23, 24]. PD-1 is expressed on T and B cells,
as well as occasionally on antigen-presenting cells (APCs) [25]. PD-1 has two ligands,
PD-L1 (expressed on almost all cell types) and PD-L2 (expressed on APCs and B cells).
PD-1 is up-regulated by antigen stimulation, while inhibitory ligands are induced
by interferons [25]. Because antigen stimulation and interferon release tend to occur
simultaneously only at the site of infection, this facilitates impairment specifically
in the infected tissue and explains why T cells in the spleen or lymph node are not
impaired in ARI.
Blockade of PD-L1 or genetic depletion of PD-1 increases the functionality of virus-specific
CD8+ T cells. In infections such as HMPV or influenza, blockade also leads to faster
virus clearance [4, 26]. Other inhibitory receptors, including Lag-3, Tim-3, 2B4,
and CTLA-4 are up-regulated in response to respiratory virus infection and appear
to contribute to some degree of T-cell impairment, but thus far PD-1 appears to be
the dominant inhibitory receptor for ARI [14]. Lag-3 mediates later T-cell impairment,
but blockade of Lag-3 increases immunopathology without reducing viral titer [17].
Although other inhibitory receptors have been discovered and characterized, to date,
they have not been evaluated for a role in T-cell impairment in viral ARI.
Is T-cell impairment the same as T-cell exhaustion?
Although many of the same inhibitory receptors have been implicated in both impairment
and exhaustion, these two states differ in a few significant ways. T-cell exhaustion
is defined by antigen-unresponsive T cells after a prolonged antigenic stimulus, such
as during chronic infections or cancer. Inhibitory receptor blockade and other strategies
to restore T-cell function are licensed strategies for cancer treatment and are in
clinical trials for chronic infection. T-cell exhaustion has been well characterized
using the lymphocytic choriomeningitis virus (LCMV) model of chronic infection and
is associated with progressive hierarchical loss of T-cell function [27] as well as
virus persistence. Pulmonary T-cell impairment is also associated with dysfunction
of multiple T-cell capacities (IL-2, TNF, granzyme B, and IFNγ production, and degranulation
as measured by CD107a,) and defects in virus clearance [4].
However, one major difference between T-cell impairment and exhaustion is timing.
T-cell exhaustion requires long-term antigen exposure before inhibitory receptors
become up-regulated. Acute LCMV infection is associated with transient PD-1 expression,
whereas chronic LCMV infection leads to sustained PD-1 expression on epitope-specific
T cells [28]. In contrast, T-cell impairment is seen relatively early (day 7–8) in
the adaptive immune response to ARI, and PD-1 expression remains high on lung lymphocytes
weeks after virus is cleared [4]. Additionally, during T-cell exhaustion, there are
subsets of exhausted T cells that can be rescued by anti–PD-1 blockade or that remain
terminally differentiated and unresponsive to PD-1 blockade [29, 30]. It is not yet
known whether similar subsets of impaired T cells exist in the lung.
It seems likely that the particular inflammatory environment of the pulmonary tissue
triggers the cascade of PD-1 and PD-L1/2 up-regulation more rapidly, accounting for
a level of impairment at day 7 of acute infection that by microarray bears a striking
similarity to exhaustion at day 30 of chronic LCMV [14, 28]. For instance, alveolar
macrophages express high levels of PD-L1 even at baseline [31], which may allow the
lung to rapidly trigger impairment, whereas induction of PD-L1 on other APCs requires
infection and inflammation.
How might our knowledge of T-cell impairment impact drug or vaccine design?
It is important to consider lung T-cell impairment when developing vaccines or therapeutics
for respiratory viruses as well as anticipating potential side effects of PD-L1 antibodies
and other checkpoint blockade therapies for cancer.
There are no licensed vaccines against many common respiratory viruses (including
RSV, HMPV, and parainfluenza viruses) despite extensive research. Vaccines that elicit
T-cell immunity are thought to enhance protection against respiratory viruses, but
whether T-cell impairment contributes to the failure of successful vaccine development
in humans requires further study. The vaccine adjuvant alum induced high PD-1 expression
on CD8+ T cells in a mouse model of influenza [32], and virus-like particle vaccination
of mice against HMPV did not protect from CD8+ T-cell impairment and inhibitory receptor
expression in secondary challenge [33]. T-cell impairment may represent a barrier
to vaccination for ARI, but pharmacologic restoration of T-cell function can have
detrimental effects in the lungs as well.
A rare but serious and often fatal side effect of checkpoint inhibitors is pneumonitis,
or inflammation of the lung [34]. Although an exact mechanism has not been established,
aberrant T-cell activation to self or foreign antigen in the lung may contribute.
Humans receiving checkpoint inhibition therapy who then acquire common respiratory
viral infections may be at increased risk for pneumonitis. A future improvement to
checkpoint therapy would be the ability to specifically target tumor-specific T cells.
Moreover, studies of respiratory virus infection in patients treated with checkpoint
inhibitors would help us understand and anticipate potential risks associated with
these treatments.
Conclusion
T-cell impairment, mediated by PD-1 and other inhibitory receptors, represents a regulatory
adaptation by the immune system to preserve healthy lung tissue during acute viral
infection. Though antibody blockade of PD-1 and other strategies to reverse T-cell
impairment offer exciting treatments for cancer, the use of these drugs can pose risks
to lung function and therefore warrant further study. Pulmonary T-cell impairment
contributes to memory and vaccine responses, though the ramifications of these interactions
are not yet clear. Finding the perfect balance between immunoprotection and immunopathology
remains elusive.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults.
Marie Griffin, Yuwei Zhu, H Keipp Talbot … (2012)

- Record: found
- Abstract: found
- Article: found
Estimates of hospitalization attributable to influenza and RSV in the US during 1997–2009, by age and risk status
Gonçalo Matias, Robert Taylor, François Haguinet … (2017)
- Record: found
- Abstract: found
- Article: not found
Vaccine adjuvants aluminum and monophosphoryl lipid A provide distinct signals to generate protective cytotoxic memory CD8 T cells.
Amy S McKee, Philippa Marrack, Megan MacLeod … (2011)