- Record: found
- Abstract: found
- Article: found
Formulation and in vitro characterization of rifampicin-loaded porous poly (ε-caprolactone) microspheres for sustained skeletal delivery
Abstract
Purpose
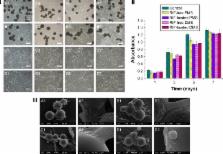
Mycobacterium tuberculosis is a serious public health problem affecting hundreds of millions of elderly people worldwide, which is difficult to be treated by traditional methods because of the peculiarity of skeletal system and liver damage caused by high-dose administration. In this research, a porous drug release system has been attempted to encapsulate rifampicin (RIF) into poly (ε-caprolactone) (PCL) microspheres to improve the efficacy and benefit of anti-tuberculosis drug in skeletal system.
Materials and methods
The microspheres prepared by two different methods, oil-in-oil (o/o) emulsion solvent evaporation method and oil-in-water (o/w) method, were characterized in terms of morphology, size, encapsulation efficiency, drug distribution, degradation, and crystallinity.
Results
The microspheres exhibited a porous structure with evenly drug distribution prepared by o/o emulsion solvent evaporation method, and their diameter ranged from 50.54 to 57.34 μm. The encapsulation efficiency was up to 61.86% when drug-loading content was only 1.51%, and showed a little decrease with the drug-loading content increasing. In vitro release studies revealed that the drug release from porous microspheres was controlled by non-Fickian diffusion, and almost 80% of the RIF were completely released after 10 days. The results of RIF-loaded microspheres on the antibacterial activity against Staphylococcus aureus proved that the porous microspheres had strong antibacterial ability. In addition, the polymer crystallinity had prominent influence on the degradation rate of microspheres regardless of the morphology.
Conclusion
It was an efficient way to entrap slightly water-soluble drug like RIF into PCL by o/o emulsion solvent evaporation method with uniform drug distribution. The RIF-loaded porous PCL microspheres showed the combination of good antimicrobial properties and excellent cytocompatibility, and it could generate gentle environment by PCL slow degradation.
Most cited references52
- Record: found
- Abstract: found
- Article: not found
Poly-є-caprolactone based formulations for drug delivery and tissue engineering: A review.
- Record: found
- Abstract: found
- Article: not found
Surface engineered and drug releasing pre-fabricated scaffolds for tissue engineering.

- Record: found
- Abstract: found
- Article: found
