- Record: found
- Abstract: found
- Article: found
Adrenocorticotropic hormone-independent macronodular adrenal hyperplasia with abnormal cortisol secretion mediated by catecholamines
letter
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
To the Editor,
Adrenocorticotropic hormone (ACTH)-independent macronodular adrenal hyperplasia (AIMAH)
is a rare cause of Cushing syndrome. In AIMAH, cortisol secretion is independent of
ACTH, and various hormones and/or cytokines have been thought to stimulate cortisol
secretion via the aberrant expression of adrenal receptors or the increased activity
of eutopic hormone receptors. Schorr and Ney [1] first proposed this concept, and
subsequently the ectopic expression of gastric inhibitory peptide (GIP), V2 and V3-vasopressin,
β-adrenergic, luteinizing hormone (LH)/human chorionic gonadotropin (hCG), serotonin,
and angiotensin receptors, as well as increased activity of a eutopic V1-vasopressin
receptor, have been identified in the adrenal gland [1,2]. Several genetic factors,
such as Gs α-subunit mutations associated with McCune-Albright syndrome and MC2R (ACTH
receptor gene) mutations, have also been postulated as causes of AIMAH.
A 50-year-old male was referred and admitted to our hospital due to uncontrolled hypertension.
He had suffered from hypertension for 6 years. His blood pressure was originally well
controlled for the first 5 years using a calcium channel blocker, but poorly controlled
for 1 year before he visited our hospital, despite his regular use of antihypertensive
agents. He was initially referred to the Cardiology Department and underwent cardiologic
evaluation after complaining of paroxysmal palpitation and dizziness. His 24-hour
Holter monitoring and coronary angiographic results were normal, except for several
antigen-presenting cells and a minimal coronary arterial obstruction at the middle
left anterior descending artery. He had a past history of major depression and had
been prescribed an antidepressive agent 18 months before he visited our hospital.
On physical examination, he had a moon face, marked central obesity (height, 167 cm;
weight, 77.65 kg; body mass index, 27.84 kg/m2), and multiple bruises on his extremities.
He also had prominent purple abdominal striae, and all of his morphological features
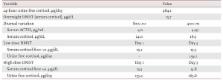
were consistent with Cushing syndrome. Laboratory examinations revealed 145.7 mEq/L
serum sodium and 2.86 mEq/L serum potassium. Arterial blood gas analysis revealed
metabolic alkalosis (pH 7.519, HCO3, 37.4 mM/L). His hemoglobin A1c level was 5.9%,
and his serum fasting blood glucose was 118 mg/dL. The results of basal endocrinological
examinations are summarized in Table 1. The circadian variation in serum cortisol
production was disrupted, and basal ACTH levels were suppressed. A 24-hour urinary
free cortisol test and overnight dexamethasone suppression test (DMST) were both suggestive
of Cushing syndrome. Low- and high-dose DMST revealed Cushing syndrome of primary
adrenal origin. An abdominal computed tomography scan and magnetic resonance imaging
(MRI) revealed bilateral large macronodular adrenal tumors. His pituitary gland was
normal on brain MRI scans. We thus diagnosed this patient with Cushing syndrome secondary
to AIMAH.
To identify aberrant receptors on the adrenal gland, we followed the investigative
protocol described by Lacroix et al. [3]. Postural and various provocation tests,
including ACTH (250 µg, intravascular), arginine vasopressin (AVP; 10 IU, intramuscular),
5-hydroxy triptamine (5-HT; 10 mg, intravascular), isoproterenol (20 ng/kg/min, intravascular
for 30 minutes) and mixed meal tests, were performed. His serum cortisol level showed
a positive response to ACTH, AVP, and isoproterenol provocation tests, but a negative
response to the postural stimulation test. The results are summarized in Fig. 1A.
If the patient had β-adrenergic or AVP receptors on his adrenal gland, then he would
have responded to the postural stimulation test; but he did not. We repeated the postural
stimulation test, and checked his endogenous antidiuretic hormone (ADH) level. He
exhibited an increased ADH level on the postural test, but no cortisol secretion (Fig.
1B).
A bilateral adrenalectomy was performed. The subsequent pathological examination of
both adrenal glands showed hyperplasia with a multinodular growth pattern: the right
and left adrenal glands were sized 14.0 × 5.0 × 3.0 cm and 9.0 × 5.0 × 3.0 cm, respectively,
and multiple golden yellow nodules measuring up to 4 cm in diameter were present.
Microscopic findings showed that the nodules consisted of variable-sized nests of
lipid-laden clear cells similar to those of the normal fasiculata layer (Fig. 2).
The final clinical and pathological diagnosis was Cushing syndrome secondary to β-adrenergic
agonist-responding AIMAH. After the operation, he took physiological doses of prednisolone
and fludrocortisone. He then lost weight gradually and achieved optimal blood pressure
with reduced doses of antihypertensive agents.
Kirschner et al. [4] first described AIMAH in 1964. They demonstrated that hypercortisolism
was ACTH-independent, and that the resected adrenal glands contained multiple nodules.
Since then, a number of cases have been described, and the cause of AIMAH has been
characterized more precisely. Previously, steroid production in AIMAH was believed
to be autonomous. In the previous study that compared the adrenal glands of patients
with AIMAH to those in patients with long-standing Cushing disease, and concluded
that prolonged adrenal stimulation by ACTH resulted in adrenal bilateral nodular formation
and varying ranges of adrenal autonomy [5]. There were also some cases in which autonomy
of the adrenal gland was the result of chronic ACTH stimulation, which eventually
resulted in ACTH suppression. However, the rarity of Nelson syndrome following bilateral
adrenalectomy in patients with AIMAH strongly argued against the adrenal autonomy
hypothesis.
In 1971, Schorr and Ney [1] first introduced the concept of aberrant adrenal receptor
expression in adrenocortical tissue. They performed in vitro studies, and found that
cyclic adenosine monophosphate and corticosterone production in rat adrenocortical
carcinoma cells were stimulated by non-ACTH hormones such as catecholamines, thyroid
stimulating hormone, follicle stimulating hormone, LH, and prostaglandin E1. This
hypothesis was later validated in humans by additional in vitro and in vivo studies.
Several ectopic receptors such as GIP, β-adrenergic receptors, vasopressin (V2-V3-vasopressin
receptor), serotonin (5-HT7 receptors), and angiotensin II receptors, and increased
expression or altered activity of eutopic receptors including the V1-vasopressin receptors,
LH/hCG receptors, serotonin (5-HT4 receptor), and leptin receptors was found [5].
In our patient, the serum cortisol level was increased by ACTH stimulation, exogenous
AVP, and isoproterenol. Because exogenous AVP could naturally stimulate ACTH and increase
serum cortisol levels, we checked serum ACTH levels during an AVP stimulation test.
His serum cortisol level was increased by 82%, and serum ACTH level was increased
by 11.5%. We were unable to confirm if AVP itself stimulated the adrenal cortex directly,
or whether the induced ACTH caused cortisol secretion. A postural test was performed
to screen for the aberrant expression of the AVP, β-adrenergic, or angiotensin II
receptors. Interestingly, cortisol secretion was stimulated by exogenous AVP and a
β-adrenergic agonist; however, a postural test failed to stimulate cortisol secretion.
We repeated the postural test, and also checked the patient's aldosterone level to
verify if the test was accurate and determined serum ADH levels to confirm if endogenous
ADH stimulated adrenal cortisol secretion. Because serum aldosterone levels increase
in response to postural stimulation, the test itself was working; however, the cortisol
response was negative. This suggests that the patient's β-adrenergic receptor showed
a blunted response to the test. Because we did not determine the serum catecholamine
levels during postural stimulation, it was unclear whether the postural test induced
sufficient endogenous catecholamine. Although a stronger stimulus (such as a treadmill
test) would have induced endogenous catecholamine and increased serum cortisol levels,
the patient refused because he had ischemic heart disease. In our case, exogenous
AVP, but not endogenous ADH, stimulated adrenal cortisol secretion. This suggests
that the cortisol response to exogenous pharmacological levels of vasopressin was
mediated by AVP-induced catecholamine release [3]. We finally concluded that this
patient had ectopic β-adrenergic receptors on the adrenal cortex, and recommended
long-term propranolol therapy. However, because he strongly desired to undergo treatment
with a rapid response, we consulted the Urological Department who recommended bilateral
adrenalectomy.
The identification of aberrant adrenal hormone receptors in AIMAH provides novel opportunities
for specific pharmacological therapies as alternatives to adrenalectomy. In 1997,
Lacroix et al. [3] reported the use of propranolol therapy for ectopic β-adrenergic
receptors in adrenal Cushing syndrome in 1997. Some studies have revealed aberrant
receptor expression in vitro using reverse transcription polymerase chain reaction.
However, a limitation of our study is that we did not confirm aberrant receptor expression
using in vitro analyses. In conclusion, we report a rare case of an AIMAH patient.
In vivo examinations suggested that altered cortisol regulation due to a β-adrenergic
agonist was involved in the pathogenesis of the AIMAH patient.
Related collections
Most cited references5
- Record: found
- Abstract: found
- Article: not found
Ectopic and abnormal hormone receptors in adrenal Cushing's syndrome.
A. LaCroix, N Ndiaye, J Tremblay … (2001)
- Record: found
- Abstract: not found
- Article: not found
Propranolol therapy for ectopic beta-adrenergic receptors in adrenal Cushing's syndrome.
Johanne Tremblay, G Rousseau, Nicole M. Bouvier … (1997)
- Record: found
- Abstract: not found
- Article: not found
CUSHING'S SYNDROME: NODULAR CORTICAL HYPERPLASIA OF ADRENAL GLANDS WITH CLINICAL AND PATHOLOGICAL FEATURES SUGGESTING ADRENOCORTICAL TUMOR.
M Lipsett, Martin Kirschner, David Powell (1964)