- Record: found
- Abstract: found
- Article: not found
Abrogation of IL-6-mediated JAK signalling by the cyclopentenone prostaglandin 15d-PGJ 2 in oral squamous carcinoma cells
Read this article at
Abstract
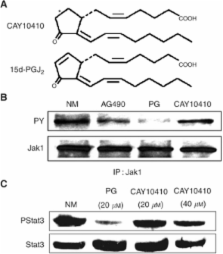
Cyclopentenone 15-deoxy-Δ 12,14-prostaglandin J 2 (15d-PGJ 2) exerts antineoplastic effects on various types of human cancer. We recently showed that treatment with 15d-PGJ 2 induces apoptosis accompanied by downregulation of the oncogenic signal transducer and activator of transcription 3 (Stat3) signalling in human oral squamous cell carcinoma (SCC) cells. The current study examines the effects of 15d-PGJ 2 on the epidermal growth factor receptor (EGFR) and Janus Kinase (JAK)-mediated signalling pathways. Inhibition of Stat3 by 15d-PGJ 2 was abolished by exogenous stimulation with transforming growth factor alpha (TGF- α), but not interleukin 6 (IL-6), supporting a selective effect of 15d-PGJ 2 on IL-6-mediated signalling. Importantly, 15d-PGJ 2 selectively abrogated constitutive and IL-6-mediated JAK phosphorylation without affecting EGFR-activated levels. Moreover, the inhibitory effect of 15d-PGJ 2 on JAK signalling required the reactive α, β-unsaturated carbon within the cyclopentenone ring. Targeting of JAK signalling using a specific JAK inhibitor also abolished Stat3 phosphorylation and resulted in apoptosis in oral SCC cells. Our findings provide the first evidence for 15d-PGJ 2–mediated downregulation of constitutive and IL-6-induced JAK signalling in cancer and support that JAK inhibition and suppression of EGFR-independent Stat3 activation by 15d-PGJ 2 represent a promising approach for induction of apoptosis in oral SCC cells.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention.
- Record: found
- Abstract: found
- Article: not found
The role of STATs in transcriptional control and their impact on cellular function.
- Record: found
- Abstract: found
- Article: not found
