- Record: found
- Abstract: found
- Article: found
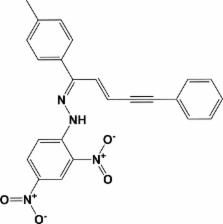
Crystal structure of ( E)-1-(2,4-dinitrophenyl)-2-[( E)-5-phenyl-1-( p-tolyl)pent-2-en-4-yn-1-ylidene]hydrazine
Read this article at
Abstract
In the title compound, C 24H 18N 4O 4, the plane of the phenyl ring is inclined to those of the toluene ring and the dinitro-substituted benzene ring by 66.96 (19) and 47.06 (18)°, respectively, while the planes of the two benzene rings are inclined to one another by 36.26 (19)°. There is an intramolecular N—H⋯O hydrogen bond between the NH group and the O atom of a nitro group, forming an S(6) ring motif. In the crystal, molecules are linked by C—H⋯O hydrogen bonds and C—H⋯π interactions, forming a three-dimensional network. There are also weak π–π interactions present involving the phenyl ring and the dinitro-substituted benzene ring [inter-centroid distance = 3.741 (2) Å].