- Record: found
- Abstract: found
- Article: found
A study demonstrating users’ preference for the adapted-REQUITE patient-reported outcome questionnaire over PRO-CTCAE ® in patients with lung cancer
Read this article at
Abstract
Introduction
The use of patient-reported outcomes (PROs) has been shown to enhance the accuracy of symptom collection and improve overall survival and quality of life. This is the first study comparing concordance and patient preference for two PRO tools: Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE ®) and the adapted-REQUITE Lung Questionnaire.
Materials and Methods
Patients with lung cancer were recruited to the study while attending outpatient clinics at a tertiary cancer centre. Clinician-reported outcomes were generated through initial patient assessment with CTCAE v4.03. Participants then completed the PRO-CTCAE ® and adapted-REQUITE questionnaires. Concordance between the 2 questionnaires was assessed by calculating Pearson correlation coefficient. PRO-CTCAE ® and CTCAE concordance was demonstrated by calculating Pearson correlation coefficient from the linear predictors of an ordinal logistic regression. P-values were also calculated.
Results
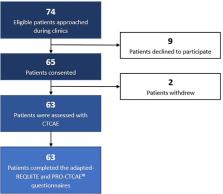
Out of 74 patients approached, 65 provided written informed consent to participate in the study. 63 (96.9%) patients completed both PRO-CTCAE ® and adapted-REQUITE questionnaires. Pearson correlation coefficient between PRO tools was 0.8-0.83 (p <.001). Correlation between CTCAE and PRO-CTCAE ® ranged between 0.66-0.82 (p <.001). Adapted-REQUITE and CTCAE correlation was higher for all symptoms ranging between 0.79-0.91 (p <.001). Acceptable discrepancies within one grade were present in 96.8%-100% of symptom domains for REQUITE and in 92.1%-96.8% for all domains in the PRO-CTCAE ®. 54% of the total participant cohort favored the adapted-REQUITE questionnaire due to reduced subjectivity in the questions and ease of use.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L)
- Record: found
- Abstract: not found
- Article: not found
Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†
- Record: found
- Abstract: found
- Article: not found