- Record: found
- Abstract: found
- Article: found
2116. Efficacy of CD388, a Novel Drug Fc-Conjugate (DFC), is Driven by the Small Molecule Neuraminidase Inhibitor (NAI)
Read this article at
Abstract
Background
Influenza prevention remains a significant public health concern that is still not adequately addressed by vaccines or current therapeutic options. Cidara has developed CD388, a multivalent conjugate of a dimeric NAI with a proprietary hIgG1 Fc domain engineered for extended half-life. CD388 is in clinical development (NCT05285137 and NCT05523089) for the prevention of seasonal influenza A and B. Herein we analyze the contribution of Fc-mediated immune effector function to CD388 efficacy.
Methods
CD388, Fc engineered to extend PK, and closely related Fc modified analogues (immune-active ‘IA’ or immune-silent ‘IS’) were generated. CD388-IA can engage Fc gamma receptors (FcγRs) whereas IS fails to engage FcγRs required to trigger Fc-mediated immune effector functions (e.g. antibody-dependent cellular cytotoxicity). Efficacy studies were conducted in BALB/c WT or Fcer1g -/- mice. The Fcer1g -/- mice are deficient in activating FcγRs thereby excluding contribution of Fc-mediated immunity to efficacy. After lethal challenge with influenza A virus, CD388 or analogues were administered subcutaneously or intramuscularly two hours post-infection. Animals were monitored daily for 14 days or longer for survival (< 20% BW loss). For viral burden quantification, lungs were harvested on day 4 post-infection and viral titers were determined by plaque assay.
Results
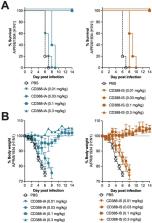
CD388-IA and -IS at comparable drug to antibody ratios (DARs) of 4.5±0.5 (used in the clinical candidate) demonstrated comparable protection (Figure 1) and comparable dose-dependent viral burden and cytokine reduction in a lethal mouse model (Table 1). Additionally, the CD388-IA analogue at DAR 4.7 was protective in Fcer1g -/- mice at the same doses required for protection in WT mice. However, a CD388-IA analogue at low DAR of 1, that has reduced antiviral activity, demonstrated improved efficacy in WT mice as compared to KO mice (Figure 2).

Efficacy of CD388-IA and CD388-IS against A/PR/8/1934 (H1N1) in a lethal mouse model in BALB/c WT mice showing survival (A) and body weight (B)
Conclusion
These data combined demonstrate that the efficacy of CD388 at DAR 4.5±0.5 is driven predominantly by the intrinsic antiviral activity of the small molecule NAI and is independent of the contribution of Fc-mediated effector functions.
Disclosures
Simon Döhrmann, PhD, Cidara Therapeutics: Stocks/Bonds James Levin, PhD, Cidara Therapeutics: Stocks/Bonds Elizabeth Abelovski, B.S., Cidara Therapeutics: Ownership Interest Amanda Almaguer, Bachelors, Cidara Therapeutics: Stocks/Bonds Rajvir Grewal, n/a, Cidara Therapeutics: Ownership Interest Karin Amundson, BSc, Cidara Therapeutics: Stocks/Bonds Joanne Fortier, BSc, Cidara Therapeutics: Stocks/Bonds Thanh Lam, PhD, Cidara Therapeutics: Stocks/Bonds Thomas P. Brady, Ph.D., Cidara Therapeutics: Stocks/Bonds Allen Borchardt, PhD, Cidara Therapeutics: Stocks/Bonds Jason N. Cole, Ph.D., Cidara Therapeutics: Stocks/Bonds Leslie W. Tari, Ph.D., Cidara Therapeutics: Stocks/Bonds

