- Record: found
- Abstract: found
- Article: found
Brazilian guidelines for allergen immunotherapy in the treatment of allergic rhinitis
research-article
Fernando Monteiro Aarestrup
1 ,
Geórgia Véras de Araújo Gueiros Lira
1 ,
Ernesto Akio Taketomi
1 ,
Elaine Gagete
1 ,
Nelson Augusto Rosário Filho
2 ,
Maria Cândida Rizzo
3 ,
Dirceu Solé
4 ,
Norma de Paula Motta Rubini
5 ,
Emanuel Savio Cavalcanti Sarinho
5 ,
Wanderley Marques Bernardo
6
,
*
02 June 2023
2023
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
INTRODUCTION
Epidemiological studies show that allergic rhinitis (AR) is observed in 10–40% of
the world's population. This disease significantly compromises the quality of life,
impairing development in children and professional activities in adults. AR is also
frequently associated with allergic asthma (AA)
1,2
. It has been observed that 15–38% of patients with AR develop concomitant AA. This
relationship between AR and AA is based on robust pathophysiological mechanisms, which
are consistent with the united airways theory. This model states that environmental
exposure to allergenic molecules in genetically predisposed individuals directs the
production of specific cytokines responsible for the development of the allergic inflammatory
process in the nasal mucosa and lungs
1,3
.
The association between AR and AA or atopic dermatitis (AD) is very common, usually
developing since childhood, representing a phenomenon called the atopic march. Therefore,
patients with AR should be evaluated in a broad and systemic way due to the implications
and interactions of this disease that is part of a broad allergic process that can
affect the upper airways, lower airways, skin, and mucous membranes. These diseases,
classified as atopic diseases, are characterized by the presence of a specific, genetically
directed immune response after exposure to allergens
1,2,4,5
. In Brazil, the components derived from the house dust mites Dermatophagoides farinae
(Df), Dermatophagoides pteronyssinus (Dp), and Blomia tropicalis (Bt) are the main
allergens associated with the etiology of AR. Particularly in southern Brazil and
in rural areas, pollens are also allergens associated with the etiology of AR
6
.
Knowledge of the pathophysiology of AR is important for understanding the diagnostic
strategies and therapeutic possibilities. Sensitization in the nasal mucosa starts
with the presentation of allergens by antigen-presenting cells, such as dendritic
cells, macrophages, and Langerhans cells, to naive CD4+ T lymphocytes, which at the
level of innate immunity may present themselves as dysfunctional, and individuals
with genetic predisposition in the presence of allergens have a tendency to differentiate
naive CD4+ T cells into Th2 cells, which are characterized by producing interleukin
(IL)-4, IL-5, and IL-13. In addition, other important cytokines in this allergen-specific
response or even in nonspecific triggers (irritants, pollutants, virus infection,
etc.) are IL-25, IL-33, and thymic stromal lymphopoietin produced by respiratory mucosal
epithelial cells. These cytokines (alarmins) can contribute to induce immunoglobulin
E (IgE) production and the recruitment of eosinophils to the site of the inflammatory
allergic process by stimulating, respectively, IL-4- and IL-5-producing Th2 and ILC2
cells. This entire process is currently referred to as type 2 inflammation, characterizing
the pathophysiological mechanisms of AR and AA
5,6
.
The Allergic Rhinitis and its Impact on Asthma (ARIA) guideline was an initiative
during a World Health Organization workshop in 1999 that established guidelines for
the treatment of AR based on allergy testing and therapeutic approach using evidence-based
medicine strategies (Grading of Recommendations, Assessment, Development and Evaluation
[GRADE] Approach). The ARIA recommendations state that allergen immunotherapy (AIT)
represents one of the cornerstones in the treatment of AR with a level of evidence
of A. The guidelines of the European Academy of Allergy and Clinical Immunology (EAACI),
World Allergy Organization (WAO), and the American Academy of Allergy, Asthma and
Immunology (AAAAI) until 2022 represented the main official documents establishing
guidelines for the use of AIT. Recently, the “position paper” of the Brazilian Association
of Allergy and Immunology (ASBAI)
6
was published, establishing recommendations for good AIT practices in Brazil. Most
of the consensus in the field considers AIT to be the unique treatment capable of
modifying the allergen-specific immune response by promoting desensitization and a
state of tolerance. The control of AR symptoms remains satisfactory in the long term
even after the end of the AIT, reducing or even abolishing the use of drugs. Therefore,
we can consider this therapy potentially able to promote total remission of the disease
1,5,6,7,8,9
.
The present study aimed to contribute to the Guidelines Project, an initiative of
the Brazilian Medical Association. Through evidence-based medicine strategies, we
conducted a systematic review in order to guide and standardize management and procedures
on the use of AIT in the treatment of AR. Clinical issues on the selection of patients
eligible for treatment with AIT through clinical history, allergy testing and/or serum-specific
IgE, information on safety and efficacy, indications and contraindications, monitoring
treatment, routes of application, and considerations on adequate professional preparation
were addressed and discussed.
METHODS
Members of the Scientific Department of Immunotherapy of the ASBAI conducted a systematic
review of randomized clinical trials (RCTs) for the construction of medical guidelines
on the use of sublingual and subcutaneous immunotherapy with dust mites and pollens
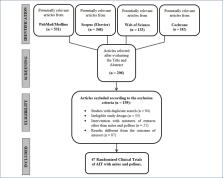
in AR. Figure 1 shows flow diagram of the RCT selection process by the Preferred Reporting
Items for Systematic Reviews and Meta-Analyses (PRISMA).
Figure 1
Flow diagram of the randomized clinical trial selection process by Preferred Reporting
Items for Systematic Reviews and Meta-Analyses
The research methods and criteria are available in the International Prospective Register
of Systematic Reviews (PROSPERO) protocol with registration number CRD42022383864;
the data from the studies were qualitatively evaluated following the PRISMA guidelines.
ELIGIBILITY CRITERIA
Inclusion criteria were defined following the P.I.C.O.S. framework. Studies that met
these criteria were eligible.
Population: patients diagnosed with persistent and/or moderate-to-severe AR (ARIA
criteria) aged >2 years.
Intervention: standard treatment (ARIA) with AIT with dust mites or pollens or standard
treatment without AIT.
Comparator: standard treatment with AIT and without AIT.
Outcomes: for the primary endpoint, we evaluated symptom reduction with clinical improvement
of rhinitis.
Study type: RCTs published in the past 30 years until November 2022, in English, Portuguese,
and Spanish languages.
SEARCH STRATEGY AND STUDY SELECTION
Searches were performed in MEDLINE/PubMed, Web of Science, Scopus, and Cochrane Library
databases for articles published until November 30, 2022, using the following descriptors,
through the Medical Subject Headings tool, in the same search protocol: for subcutaneous
immunotherapy with dust mites: “allergic rhinitis” AND “allergen immunotherapy” AND
“house dust mite extracts” AND “subcutaneous”; for sublingual immunotherapy with dust
mites: “allergic rhinitis” AND “allergen immunotherapy” AND “house dust mite extracts”
AND “sublingual”; for subcutaneous immunotherapy with pollens: “allergic rhinitis”
AND “allergen immunotherapy” AND “pollens extracts” AND “subcutaneous”; and for sublingual
immunotherapy with pollens: “allergic rhinitis” AND “allergen immunotherapy” AND “pollens
extracts” AND “sublingual.”
DATA EXTRACTION AND SYNTHESIS
Quality assessment was obtained using the GRADE approach to assign levels of evidence
and rate the strength of recommendation of the results. The quality of evidence was
classified into four levels: high, moderate, low, and very low. The following factors
were considered to determine the level of evidence: study design, methodological limitations
(risk of bias), inconsistency, imprecision, and magnitude of effect. After this analysis,
the strength of the recommendation was identified as weak or strong, and an evaluation
of the clinical trials was performed together.
For risk of bias assessment, the revised Cochrane Risk of Bias (RoB2) tool was used
for selected randomized trials. RoB2 was judged as low, moderate, high, or unclear
for each domain: randomization process, deviations from intended interventions, lack
of outcome data, outcome measurement, selection of reported outcomes, and overall
bias. The domains included in this tool were divided according to the phase of the
intervention: pre-intervention (bias due to confounding, bias in selection of participants
for the study), intervention (bias in classification of interventions), and post-intervention
(bias due to deviations from intended interventions, bias due to lack of data, bias
in measurement of outcomes, and bias in selection of reported outcomes).
CLINICAL QUESTIONS: EVIDENCE ANALYSIS
Tables 1, 2, and 3 present the data analysis of the risk of bias and grading of the
value of evidence by the GRADE approach. In each clinical question answered below,
these analyses were taken into account to establish the conclusions and recommendations.
The GRADE analysis was performed using the set of articles analyzed specifically for
house dust mites and pollens.
Table 1
RoB2 analysis to house dust mite allergen immunotherapy.
Intention-to-treat
Unique ID
Study ID
D1
D2
D3
D4
D5
Overall
1
Bahçeciler - 2001
2
Bergmann - 2014
3
Bernstein - 2018
4
Bozek - 2013
5
Chen - 2020
6
De Bot - 2012
7
Demoly - 2021
8
Di Gioacchino - 2012
9
Didier - 2015
10
Dokic - 2005
11
Guez - 2000
12
Karakoc-Aydiner - 2015
13
Masuyama - 2019
14
Mosbech - 2015
15
Okamoto - 2017
16
Okamoto - 2019
17
Riechelmann - 2010
18
Tonnel - 2004
19
Tseng - 2008
20
Valero - 2022
21
Varney - 2003
22
Vesna - 2016
23
Xian - 2019
24
Yu Guo - 2017
25
Yukselen - 2013
Low risk
Some concerns
High risk
D1
Randomisation process
D2
Deviations from the intended interventions
D3
Missing outcome data
D4
Measurement of the outcome
D5
Selection of the reported result
Table 2
RoB2 analysis to pollens allergen immunotherapy.
Intention-to-treat
Unique ID
Study ID
D1
D2
D3
D4
D5
Overall
1
Ahmadiafshar - 2012
2
Bowen - 2004
3
Bozek - 2020
4
Bufe - 2004
5
Clavel - 1998
6
Couroux - 2019
7
De Blay - 2007
8
Durham - 2012
9
Gotoh - 2019
10
Lou - 2020
11
Nolte - 2020
12
Nolte - 2021
13
Okamoto - 2015
14
Pfaar - 2008
15
Pfaar - 2010
16
Pfaar - 2019
17
Sharif - 2019
18
Ünal - 2020
19
Wahn - 2012
20
Worm - 2019
21
Yang - 2022
22
Yonekura - 2021
Low risk
Some concerns
High risk
D1
Randomisation process
D2
Deviations from the intended interventions
D3
Missing outcome data
D4
Measurement of the outcome
D5
Selection of the reported result
Table 3
GRADE analysis.
Question: Dp and Df mite extracts compared to Placebo with the same organoleptic characteristics
for persistent and/or moderate-to-severe allergic rhinitis (ARIA criteria)
Context: To evaluate the reduction of symptoms with clinical improvement in allergic
rhinitis.
Certainty assessment
Number of patients
Certainty
Number of studies
Study design
Risk of bias
Inconsistency
Indirect evidence
Imprecision
Other considerations
Dp and Df mite extracts
Placebo with the same organoleptic characteristics
25
Randomized clinical trials
Nonsevere
Nonsevere
Nonsevere
Nonsevere
None
4.518
3.887
⨁⨁⨁⨁ High
Question: Grass pollen extract compared to placebo for perennial or seasonal allergic
rhinitis
Context: To evaluate the reduction of symptoms with clinical improvement in allergic
rhinitis.
Certainty assessment
Number of patients
Certainty
Number of studies
Study design
Risk of bias
Inconsistency
Indirect evidence
Imprecision
Other considerations
Grass pollen extracts
Placebo with the same organoleptic characteristics
22
Randomized clinical trials
Severe
Nonsevere
Nonsevere
Nonsevere
None
2.945
2.248
⨁⨁⨁◯ Moderate
Question 1: Is subcutaneous allergen immunotherapy effective in allergic rhinitis
in children and adults?
The clinical picture of AR may present in seasonal or perennial clinical form, caused
respectively by pollen/fungi and house dust containing predominantly components derived
from house dust mites, animal epithelia, and fungi
2,6,7,10–17
.
In cases of moderate-to-severe persistent AR, AIT, administered by sublingual (SLIT)
or subcutaneous (SCIT) route, is a therapeutic modality considered one of the pillars
of the professional practice of the specialist in allergy and immunology. AIT has
shown to be effective, contributing significantly to clinical improvement by reducing
symptom scores and medication use, whose effects may persist for several years after
discontinuation (termination). Thus, the etiologic diagnosis of AR responsible for
IgE antibody-mediated sensitization, determining its clinical relevance, is crucial
for the allergist with RQE (specialty qualification record) doctor in allergy and
immunology and/or pediatric allergy practice area to carry out the selection (formulation)
of allergenic extract components and their use in different dilutions in an appropriate
manner for the proper choice of route of administration, whether subcutaneous or sublingual,
and its application scheme (protocol). Also, it is of fundamental importance to know
the properties of the allergens so that the specialist can choose whether or not to
mix certain allergens in cases of polysensitized patients
6,18–26
.
This systematic review included 25 double-blind, placebo-controlled (DBPC) RCTs with
a total of 4,518 patients with perennial AR with or without asthma who underwent immunotherapy
with house dust mites (Dermatophagoides pteronyssinus and Dermatophagoides farinae,
in a 1:1 ratio) and 3,887 placebo-treated control patients, and when analyzed by the
GRADE approach (Table 3; classification of recommendations, assessment, development,
and evaluation) showed a level of CERTAINTY considered HIGH, with no seriousness detected
in the parameters of risk of bias, inconsistency, indirect evidence, and imprecision,
as shown in Table 3 (GRADE for RCTs involving AIT with dust mites). Among the total
of these 25 RCTs, 3 studies involved three comparative groups: SCIT, SLIT, and placebo/control
(pharmacotherapy only, in the study conducted by Karakoc-Aydiner)
15
, and the rest employed only one active treatment modality. Thus, in all seven trials
(four trials with SCIT active group and placebo; three trials with SCIT and SLIT active
groups versus control) that employed ITSC, they demonstrated clinical efficacy in
the treatment of AR by reducing symptom and/or medication scores compared to the placebo
group, as shown in Table 1 (RoB2, AIT with dust mite allergens).
For AIT with grass or tree pollen allergens, 22 DBPC RCTs were included, with a total
sample size of 2,945 patients with seasonal AR receiving grass or tree pollen immunotherapy
and 2,248 patients in the placebo group. Analyzing these trials, because they are
the most heterogeneous trials, the joint analysis of these trials by GRADE (Table
3) showed a level of CERTAINTY considered MODERATE, although it was not detected any
severity in the parameters of inconsistency, indirect evidence, and imprecision, but
showed serious risk of bias, as can be seen in Table 3 (GRADE of RCTs with pollens).
Of the total of these 22 RCTs, 5 employed SCIT, and 17 SLIT, as shown in Table 2 (RoB2,
AIT with pollen allergens).
Conclusions
SCIT with house dust mites is effective in AR in children and adults (GRADE: high;
GRADE OF RECOMMENDATION: strong).
SCIT with pollens is effective in AR in children and adults (GRADE: moderate; RECOMMENDATION:
strong).
Question 2: Is subcutaneous immunotherapy safe in allergic rhinitis in children and
adults?
Despite the evidence of beneficial clinical effect of SCIT, this therapeutic modality
presents risks of developing adverse effects, either in children or adults, especially
local reactions such as discomfort, erythema, edema, pain, and pruritus at the application
site, usually of mild intensity. Local treatment can be given for these local reactions
with cold/iced compresses and/or topical corticosteroids or oral antihistamines. However,
patients with frequent and extensive local reactions should be treated with caution,
as they may be at greater risk of systemic reactions. In this context, systemic adverse
effects may occur, mostly mild, including sneezing, pruritus, nasal congestion, and/or
urticaria, which are easily controlled and are not troublesome for the continuation
of immunotherapy. In patients with AR and concomitant asthma, it is always recommended
to evaluate the acute exacerbation of asthma and measure the peak flow before the
application of SCIT, and it should be suspended in the presence of acute asthmatic
symptoms. In addition, the greatest concern should be directed toward the serious
systemic adverse effects, which, although rare, can occasionally present anaphylaxis
and even death has been reported in the literature. Thus, for SCIT, applications require
a location with appropriate infrastructure
18
, according to the Annex of Resolution CFM 2.215/2018 (Federal Medical Council), and
immediate medical care. In cases of anaphylaxis, the treatment of choice is intramuscular
application of millesimal epinephrine/adrenaline. Antihistamines and systemic corticosteroids
are considered secondary medications. It is recommended that the site of the SCIT
should be at the prescribing physician's facility
2,6,9,14
.
In addition, Purkey et al.
21
in their evidence-based review recommended the use of SCIT for patients with AR, whether
seasonal or perennial, especially for those who are not responsive to usual pharmacological
therapy and whose symptoms significantly impact their quality of life. These authors
stated that SCIT is safe when administered carefully to specific patients and applied
in settings capable of providing appropriate medical care in the event of systemic
adverse reactions.
Conclusions
SCIT with house dust mites is safe in AR in children and adults (GRADE: high; GRADE
OF RECOMMENDATION: strong).
SCIT with pollens is safe in AR in children and adults (GRADE: moderate; GRADE OF
RECOMMENDATION: strong).
It is recommended that SCIT should be performed at the prescribing physician's facility.
The application must always be performed under medical supervision in a place with
adequate infrastructure to attend eventual systemic adverse reactions
2,6
.
Question 3: Is sublingual immunotherapy effective in allergic rhinitis in children
and adults?
Due to its clinical efficacy and high safety, SLIT, initially approved by European
health surveillance agencies, particularly in Italy, has spread its use all over the
world, including countries in the East, such as Japan, China, and Australia; North
America, such as the United States and Canada; and several countries in South America,
especially Brazil.
Among the 25 RCTs employing AIT with dust mite allergens used in this systematic review
shown in Table 1 (RoB2, AIT with dust mite allergens), 21 clinical trials used SLIT
containing a proportional mixture of the dust mites Dermatophagoides pteronyssinus
and Dermatophagoides farinae, all of which showed clinical efficacy by reducing symptom
and/or medication scores when compared to the placebo group, except in a study by
Karokoc-Aydiner
15
which was found to have reduced symptoms in both the intervention group and the placebo
group. Interestingly, 12 trials employed SLIT in the form of sublingual drops, and
9 studies used SLIT in the form of tablets. Thus, the data from most well-controlled
clinical trials have demonstrated that SLIT is indeed effective in treating AR in
both children and adults, not only in its short-term use (12 months), but also in
its long-term use (up to 3 years in the active group). Therefore, it has been well
documented through controlled double-blind trials that SLIT is capable of inducing
modifying effects on the natural course of the disease, particularly when SLIT is
employed with grass pollens, since the duration of its effects lasts for at least
2 years after a 3-year treatment period
24
. Its preventive effect should also be taken into consideration, since children and
adolescents with AR treated with SLIT are found to have less chance of developing
asthma later, that is, this intervention has altered the atopic march. Due to its
beneficial effects, SLIT with house dust mites has been registered and authorized
as a drug/medication by health surveillance agencies
25
.
Among the 22 RCTs using grass or tree pollens presented in Table 2, 17 trials used
SLIT, 10 of which were in the form of sublingual drops, 6 in tablet form, and 1 in
spray form. Also, SLIT with grass and tree pollens has been shown to be effective,
whether employing a continuous or noncontinuous regimen. In the latter type, the period
of SLIT administration can be on a pre-seasonal, pre-co-seasonal, or seasonal regimen.
Meta-analysis studies, where a set of patients are analyzed by different investigators,
have shown that SLIT with grass extract in pre-co-seasonal regimens has progressively
reduced the combined symptom and medication scores over the course of treatment, a
reduction from 29% in the 1st year to 45% in the 3rd year of treatment. It has also
been noted that the clinical efficacy of using SLIT with pollens can be seen from
the first month of treatment
25
.
Conclusions
SLIT with house dust mites is effective in AR in children and adults (GRADE: high;
GRADE OF RECOMMENDATION: strong).
SLIT with pollens is effective for AR in children and adults (GRADE: moderate; RECOMMENDATION:
strong).
Question 4: Is sublingual immunotherapy safe in allergic rhinitis in children and
adults?
SLIT is generally well tolerated, even at high doses, with good clinical safety
27–45
. In the vast majority of patients undergoing SLIT, the predominant adverse effects
are mild or moderate oral reactions, such as itching, and mouth and throat irritation.
Many of these effects are observed early in the course of treatment (in the induction
phase). Tingling sensation (oral paresthesia), lip edema, tongue edema, glossodynia,
dysgeusia, abdominal pain, diarrhea, and headache have also been reported. Coughing
and dyspnea are likely to occur in patients who have AR concomitant with asthma
26,36,40,42
. It is important to know that mild adverse effects are relatively frequent, with
studies showing that 46–69% of the patients treated with SLIT with grasses have reported
that the adverse effects were directly linked to the treatment. In this regard, 5%
of patients have discontinued treatment due to adverse effects secondary to SLIT.
Radulovic et al.
3
performed a meta-analysis of 60 clinical trials of SLIT in patients with AR with or
without asthma, and the overall interpretation was that SLIT was shown to be quite
safe, showing predominantly mild-to-moderate local reactions with no need for treatment
in numerous studies, but there were no serious adverse reactions, and no patients
required the use of adrenaline. Thus, the authors considered that analyses of adverse
events were crucial, giving the advantage of SLIT as an alternative to SCIT for its
low incidence of systemic adverse effects. Local reactions are common in SLIT with
seasonal or perennial allergens compared to the placebo group, and these effects are
unavoidable but are generally seen as an inconvenience that cause little distress
and have no lasting effect, although some effects may be distressing enough to abandon
treatment. Systemic reactions are largely confined to the upper respiratory tract
and associated organs (rhinitis, conjunctivitis, or rhinoconjunctivitis), with these
occurring more frequently in the SLIT group than in the placebo group. Gastrointestinal
effects occur predominantly in pediatric patients, but no reactions were considered
serious. Importantly, no serious systemic reaction, anaphylaxis, or death was observed
in this meta-analysis.
Di Bona et al.
27
, in their systematic review and meta-analysis, found the occurrence of adverse events
in 1,384 (61.3%) of 2,259 adult and child patients who received SLIT with grass pollen
allergens and in 477 (20.9%) of 2,279 patients in the placebo group. In addition,
seven patients in the SLIT group were reported to have had adverse events related
to immunotherapy that required the application of epinephrine. The authors concluded
that the findings showed little benefit of SLIT with grass pollen tablets for reducing
symptom and medication (antihistamines and corticosteroids) scores in patients with
seasonal allergic rhinoconjunctivitis, and thus, due to the small benefit, these authors
opined that convenience and ease in its administration do not seem to be sufficient
reasons for choosing this route.
It should be noted that the EAACI guidelines recommend both routes of administration,
subcutaneous or sublingual, for the treatment of AR or allergic rhinoconjunctivitis,
perennial, or seasonal, in children or adults. The allergic disease should necessarily
be mediated by IgE antibodies to clinically relevant allergens in one or more allergen
groups, especially in patients with moderate or severe allergy, whose symptoms affect
the quality of life or nighttime sleep
27–29
. It is crucial to know and keep in mind that the recommendations for good clinical
practice in AIT from the ASBAI are in agreement with these EAACI guidelines
6,9
. However, the data needed to determine which route of administration is more effective,
subcutaneous or sublingual, are currently insufficient
29
. Therefore, each specialist in Allergy and Immunology should carefully analyze each
case individually, using their technical and scientific knowledge, and, together with
the patient or caregiver, choose and decide on the best route of administration of
AIT.
Conclusions
SLIT with house dust mites is safe for AR in children and adults (GRADE: high; RECOMMENDATION
GRADE: strong).
SLIT with pollens is safe for AR in children and adults (GRADE: moderate; RECOMMENDATION:
strong).
Question 5: What are the criteria for indicating allergen immunotherapy in allergic
rhinitis?
AR can be classified in terms of frequency into intermittent and persistent, and in
terms of intensity into mild and moderate-to-severe, according to the ARIA guidelines
1
. The so-called seasonal form, whose main characteristic is intermittence, is caused
by a mechanism of immediate hypersensitivity to allergens that are predominantly external
to the home (mainly pollens and fungi); on the contrary, persistent (perennial) rhinitis
is characterized by sensitization to in-home allergens, such as dust mites, fungi,
cockroaches, and animals.
The main criterion for AIT indication is that the rhinitis should be moderate-to-severe,
caused by an identified allergen responsible for the induction of specific IgE antibodies,
either perennial or seasonal, that is related to the patient's symptoms, and whose
drug therapy, together with specific environmental control measures, has not been
sufficient for symptom control. This criterion was used in all DBPC RCT studies analyzed
in this current systematic review. A few comments will follow.
All these studies referred to Dermatophagoides pteronyssinus and D. farinae, as shown
in Table 1 (RoB2 AIT with mites)
15,19,30–52
or regional pollens, according to Table 2 (RoB2 AIT with pollens)
12,16,24,26,53–70
, requiring more consistent studies on other common mites in our environment, such
as Blomia tropicalis, and even controlled studies with other aeroallergens, such as
fungi and epithelium from domestic animals. Nevertheless, Aria
1
as well as guidelines from AAAAI
2
, EAACI
5
, and ASBAI
6
recognized the AIT as valid when performed with other extracts, as long as they are
of good quality, preferably standardized, and with the correct mixture of allergens/antigens,
since some allergens may have proteolytic enzymes that inactivate other components
of the mixture.
Besides the diagnosis of allergic sensitization, the correlation between allergic
sensitization and the onset of symptoms is essential for the indication of AIT. In
this context, several authors have performed nasal provocation tests
15,19,33,42,45,47,66
and ocular provocation tests
44,64,67
to better characterize this association.
Regarding age, DBPC studies in young children are scarce. The minimum age reported
was 4 years for SLIT
34,67
and 5 years for SCIT
15
. Considering that SLIT is safe and easily accepted by children, the Brazilian consensus
suggests an age of 2 years as the lower limit of indication for this treatment
6
. There is no maximum age beyond which AIT cannot be used, and the contraindications
are much more due to comorbidities in this age group than the age itself. Gotoh et
al.
59
used SLIT in a large number of patients between 5 and 64 years of age. Bozek et al.
33,55
studied elderly patients up to 75 years old, attesting to the efficacy and safety
of AIT, since these contraindications are respected.
Most studies and consensus suggest the age of 65 years as the limit for AIT indication,
since the immune response decreases and the risks increase with senescence
2,5,6,24,49,65
.
Conclusions
The indications for AIT in patients with AR or allergic Rhinoconjunctivitis are as
follows:
Moderate-to-severe disease not controlled despite environmental and medication measures
or when the patient desires control without the use of medications.
Accurate diagnosis of IgE-mediated allergic sensitization through allergy testing
(prick test) and/or serum-specific IgE.
Correlation between allergic sensitization and triggering of symptoms. In practice,
this correlation is clinical and, if possible, nasal and/or ocular provocation tests
can be added; however, these procedures are more often reserved for studies.
Patients with minimum and maximum age and clinical condition compatible with the chosen
treatment (SLIT or SCIT), namely from 2 to 4 years for sublingual treatment and above
5 years for subcutaneous treatment, up to approximately 65 years old for both therapies.
Question 6: What are the absolute and relative contraindications of allergen immunotherapy
in allergic rhinitis?
SLIT has a higher safety profile than SCIT since the latter can develop systemic reactions
and even anaphylaxis, which is extremely rare in the sublingual route
46–48
. Therefore, contraindications are less restrictive in SLIT. However, in general,
the diseases listed below constitute relative or absolute impediments to indicating
both.
Severe and poorly controlled asthma
This is an absolute contraindication in all studies and consensus statements
2,4–6,8,9,12,14–17,19,20,24,26,29–70
.
AR is often associated with asthma, and it is mandatory that asthma be controlled
before AIT can be indicated. Individuals with FEV1 whose value is less than 70–80%
of baseline are not included in research protocols
15,30,32,33,38
. However, mild or moderate asthma, since it is controlled, is not an absolute contraindication
but a relative one because the risks versus benefits of the procedure have to be controlled,
particularly in AIT-SC
15,16
, although the sublingual route is more indicated for these patients
15,26,30,33,44,45,52,56,57,64–67
.
Underlying diseases
Diseases cited as contraindications to AIT are severe diseases of the immune system,
such as autoimmunities; active infectious diseases, such as tuberculosis; heart disease,
especially coronary heart disease; and any other disease that contraindicates the
use of adrenaline: severe hypertension, even if controlled; severe kidney disease;
systemic use of corticosteroids; use of beta-blockers and angiotensin-converting enzyme
(ACE) inhibitors; use of immunosuppressants; severe AD; neoplasms; psychiatric diseases
that prevent the individual from being fully conscious; lack of adherence to treatment;
and drug abuse
26,37,44,47,49,51,54,64,67
.
However, according to the main consensus
1,2,6,29
, the stage of the disease and its severity must be considered, since controlled immunological
diseases, use of ACE inhibitors, beta-blockers, and diseases in general, where the
risk of AIT is lower than its benefits, are relative contraindications.
Some studies report anatomical alterations of the upper airways and/or previous otorhinolaryngological
surgery as exclusion factors for AIT
35,46
, but these are not absolute contraindications, and the cost/benefit ratio and the
correct diagnosis of rhinitis should always be considered in these cases.
Nolte et al.
61
excluded patients with eosinophilic esophagitis for using SLIT.
Pregnancy and lactation
There is consensus among researchers that for pregnant and nursing women, AIT should
not be prescribed
19,24,26,32,37–39,41,45,47–49,51,54,57,63–66
. In this context, Guo et al.
51
have even required that patients be on contraceptives to enter in their research protocol.
However, if the patient becomes pregnant during treatment, the consensuses recommend
that treatment does not need to be discontinued, but that the allergen concentration
should not be increased if the AIT is still in induction phase
1,2,4–6,29
. This is in agreement with Mosbech et al.
41
who reported pregnancy during the course of the study without mentioning that such
patients were excluded from the study.
Conclusions
Poorly controlled asthma and severe active diseases (especially immunological, infectious,
and neoplastic) are absolute contraindications for using AIT.
Eosinophilic esophagitis is an absolute contraindication for the use of SLIT.
Controlled cardiovascular diseases, use of ACE inhibitors, beta-blockers, chronic
diseases under control, and mild psychiatric diseases are relative contraindications
where risk versus benefit must be evaluated individually.
Pregnancy and lactation are conditions that absolutely contraindicate the beginning
of treatment, but not in its continuity, when increasing the AIT concentration is
contraindicated if it is in the induction phase.
Lack of compliance should be considered as a factor to contraindicate the initiation
or continuation of the AIT.
Question 7: What are the criteria for monitoring the effectiveness of allergen immunotherapy
in allergic rhinitis?
There are simple questionnaires, where a score is assigned according to the intensity
of symptoms and need for medication, in diaries requested to the patient or caregivers,
and at regular intervals these scores are analyzed
15,19,24,26,30–32,35,39,40–46,48,49,51–54,56,57,63–67
. Several authors use the visual analog scale (VAS) standardized by ARIA
15,33,35,37,47
, in which rhinitis symptoms, such as obstruction, itching, sneezing, rhinorrhea,
and ocular symptoms, as well as the general perception of such symptoms in the quality
of life, are jointly measured on a ruler with figures, and the patient is asked to
mark his or her situation along this ruler, which ranges from 0 (totally asymptomatic)
to 10 (very bad symptoms, totally uncontrolled)
71,72
. Some authors use their own VAS, with different scores for symptoms
42,44,51,64
.
In addition, some researchers ask for an overall score for the AIT to be given at
each year of treatment where zero is where there was worsening of rhinitis after 1
year with therapy and the maximum score where there was marked improvement
31,49,51
. Studies also emphasize the need to have questionnaires for specific scoring regarding
adverse effects
32,35,37,38,40
. Quality of life questionnaires have been added in several trials
16,35,40,41
.
Currently, studies with immunological biomarkers such as IgG4 and specific IgE still
show conflicting results, and they are not used in clinical practice for monitoring
efficacy or even for treatment discontinuation, remaining restricted to the research
field. It is also important to note that the decrease in papule size in skin tests
is controversial, with some authors reporting a decrease
19,30,53
, but others not
39,47,53
. Therefore, this is not a good parameter for monitoring or for the efficacy of the
AIT.
Conclusions
Currently, the criteria for monitoring AIT are clinical, evaluating the symptom and
medication scores, preferably through the various scales provided in the consensuses.
This evaluation can be complemented with quality of life questionnaires.
Assessment of side effects should also be monitored.
There are currently no clinically available immunological biomarkers for monitoring
AIT.
Skin testing should not be performed as a means of monitoring the efficacy or duration
of the AIT.
Question 8: What are the recommendations for discontinuation of allergen immunotherapy
in allergic rhinitis?
All consensus statements
1,2,4–6,29
suggest a minimum of 3 years of duration of AIT, at least for perennial allergens,
which is necessary to have a sustained response to treatment. In fact, Durham et al.
24
continued to evaluate patients treated or not treated (control group) after the end
of SLIT during 3 years for pollens and found a significant improvement in the active
group regarding clinical scores even 2 years after the end of treatment. Chen et al.
34
observed children for three more years after 3 years of treatment with SLIT for dust
mites and likewise found sustained efficacy in the group that received active treatment.
Gotoh et al.
59
likewise obtained positive results even after 2 years of the termination of SLIT for
pollen, maintained for 3 years in the pollen seasons.
Conclusions
The optimal duration time for AIT is 3–5 years after the beginning of the maintenance
phase. AIT should be maintained for at least 3 years to achieve lasting efficacy.
In case of pollinosis, AIT can be performed only for a few months before and during
the pollen season (pre-co-seasonal regimen), although in most Brazilian regional,
allergens are perennial and not seasonal, except in the southern states.
As previously mentioned, the skin test is not a good parameter for discontinuation
of AIT, and at present, there are no laboratorial biomarkers to guide the duration
of the treatment.
Clinical evaluation is always the best parameter to assess the efficacy of AIT. In
case of lack of clinical results after reaching the maintenance dose, AIT can be discontinued.
CONCLUDING REMARKS
The main purpose of this systematic review was to establish best practice guidelines
for the use of AIT in the treatment of AR. Evidence-based medicine strategies were
used to answer relevant clinical questions. The primary endpoints investigated in
each study included in this systematic review showed a high degree of evidence for
the efficacy and safety of AIT in the treatment of AR in patients sensitized to house
dust mites, which correspond to the major allergens associated with the etiopathogenesis
of AR in Brazil. We emphasize that recognition of allergic sensitization through appropriate
allergy testing and careful clinical evaluation of patients is critical to recognize
patients with indications for allergy treatment. Since AR is one of the diseases that
is part of the atopic march, a systematic evaluation of patients should be performed,
taking into consideration the diagnosis and treatment of other atopic diseases such
as AA and AD.
The appropriate choice and management of allergenic extracts to be used in the personalized
vaccine used in the AIT is a fundamental condition for achieving the expected results
in clinical practice. In Brazil, CFM Resolution No. 2215/2018 regulates the use of
allergenic extracts for diagnostic and therapeutic purposes in allergic diseases
18
. The technical responsibility of allergy and immunology services must be exercised
by a physician with a RQE in Allergy and Immunology, in the CRM of their jurisdiction,
according to Chapter III, article 9, paragraph 1 of the Annex of CFM Resolution No.
2147/2016. In services with exclusive care of pediatric patients, the technical responsibility
must be exercised by a physician with an RQE in Allergy and Immunology or RQE of qualification
in Pediatric Allergy and Immunology.
Taken together, the data presented here allow us to make a strong recommendation for
the use of AIT, either subcutaneously (SCIT) or sublingually (SLIT) in the treatment
of AR.
AIT induces changes in the immune response and promotes symptom control in AR through
immunomodulation of the allergen-specific response. In this way, AIT allows for clinical
remission of AR for prolonged periods without the use of drugs, even after administration
has ceased. This therapeutic strategy is currently the only known way to modify the
natural history of allergic diseases. Due to the immunomodulation promoted by AIT,
patients with AR, besides benefiting from the control of symptoms through this allergen-specific
treatment, can also be preventively protected against the development of other atopic
diseases such as AA and AD.
Related collections
Most cited references81
- Record: found
- Abstract: found
- Article: not found
Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision.
- Record: found
- Abstract: found
- Article: not found
EAACI Guidelines on Allergen Immunotherapy: Allergic rhinoconjunctivitis
L. JACOBSEN, O Tsilochristou, L. Zhang … (2017)

- Record: found
- Abstract: found
- Article: found
Sublingual immunotherapy: World Allergy Organization position paper 2013 update
Giorgio Canonica, Linda Cox, Ruby Pawankar … (2014)