- Record: found
- Abstract: found
- Article: found
Update on new biologics for intractable eosinophilic asthma: impact of reslizumab
Abstract
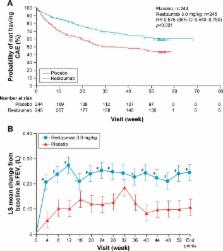
A small percentage of patients with asthma have uncontrolled symptoms and frequent exacerbations, despite treatment with inhaled corticosteroids and other agents. It has become clear that different subtypes of this severe, treatment-resistant group exist due to different mechanisms of the disease. All such patients require detailed assessment in specialist centers to characterize the disease and assess treatment adherence. Recently, monoclonal antibodies have become available, which target specific pathways that may contribute to persistent inflammation and asthma exacerbations. These antibodies include those targeting interleukin (IL)-5, which drives eosinophilic inflammation. Reslizumab is a newly licensed antibody that blocks binding of IL-5 to its receptor. Here, we discuss the significance of clinical data of this drug, which show up to 50% reduction in exacerbation rates, together with modest but significant improvements in lung function and quality of life, in those with persistent eosinophilia. The combination of reslizumab with mepolizumab and benralizumab, which also target IL-5, may be a useful addition to the therapeutic armamentarium in a selected group of patients with severe asthma.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial.
- Record: found
- Abstract: found
- Article: not found