- Record: found
- Abstract: found
- Article: not found
Longer treatment intervals are associated with reduced treatment persistence in neovascular age related macular degeneration
Read this article at
Abstract
Aims
To test the hypothesis that patients treated for neovascular age related macular degeneration (nAMD) with longer treatment intervals are more likely to persist with treatment.
Methods
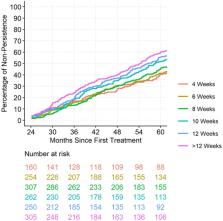
Data were obtained from the prospectively-defined Fight Retinal Blindness! registry. Treatment interval at 2 years was stratified based on the mean treatment interval over the three visits prior to and including the 2-year visit. Rates of non-persistence to follow-up were assessed from 2 to 5 years.
Results
Data from 1538 eyes were included. The overall rate of non-persistence was 51% at 5 years. Patients on longer treatment intervals (12-weeks) at 2 years were found to be less persistent to long-term follow-up. These eyes were found to have fewer active disease visits in the first 2 years (40%) than eyes treated at 4-weekly intervals (66%, p < 0.001). In the multivariable analysis, better vision at 2 years was associated with a lower risk of non-persistence (hazards ratio [HR] [95% CI]: 0.95 [0.93, 0.97], P < 0.001), while longer treatment intervals (HR [95% CI]: 1.31 [0.95, 1.8] and 1.54 [1.15, 2.06] for 12-week and > 12-week intervals vs. 4-week intervals, respectively, P = 0.002) and older patients (HR [95% CI]: 1.03 [1.02, 1.04], p < 0.001) were at higher risk of non-persistence.
Conclusions
We found that patients on longer treatment intervals at 2 years were more likely to be non-persistent with treatment in later years. Reinforcing the need for ongoing treatment is important for patients on longer intervals who may feel complacent or that treatment is no longer effective, particularly if newer, longer lasting agents become widely available.
Related collections
Most cited references25

- Record: found
- Abstract: found
- Article: found
Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA)
- Record: found
- Abstract: found
- Article: not found
Prospective Trial of Treat-and-Extend versus Monthly Dosing for Neovascular Age-Related Macular Degeneration: TREX-AMD 1-Year Results.
- Record: found
- Abstract: found
- Article: not found
