- Record: found
- Abstract: found
- Article: found
Cross-Talk between Oxysterols and Glucocorticoids: Differential Regulation of Secreted Phopholipase A2 and Impact on Oligodendrocyte Death
Read this article at
Abstract
Background
Oxysterols are oxidized forms of cholesterol. They have been shown to be implicated in cholesterol turnover, inflammation and in neurodegenerative diseases such as Alzheimer's disease and multiple sclerosis. Glial cells are targets of oxysterols: they inhibit astrocyte proliferation after brain injury, and we have previously shown that 25-hydroxycholesterol (25OH) provokes oligodendrocyte apoptosis and stimulates the expression of sPLA2 type IIA (sPLA2-IIA), which has a protective effect.
Methodology/Principal Findings
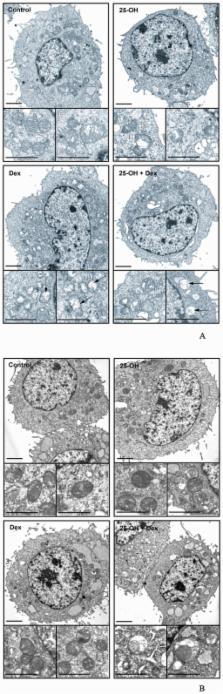
As glucocorticoids are well-known for their anti-inflammatory effects, our aim was to understand their direct effects on oxysterol-induced responses in oligodendrocytes (sPLA2-IIA stimulation and apoptosis). We demonstrate that the synthetic glucocorticoid dexamethasone (Dex) abolishes the stimulation of sPLA2-IIA by 25-hydroxycholesterol (25-OH). This inhibition is mediated by the glucocorticoid receptor (GR), which decreases the expression of the oxysterol receptor Pregnane X Receptor (PXR) and interferes with oxysterol signaling by recruiting a common limiting coactivator PGC1α. Consistent with the finding that sPLA2-IIA can partially protect oligodendrocytes against oxysterol-triggered apoptosis, we demonstrate here that the inhibition of sPLA2-IIA by Dex accelerates the apoptotic phenomenon, leading to a shift towards necrosis. We have shown by atomic force microscopy and electron microscopy that 25-OH and Dex alters oligodendrocyte shape and disorganizes the cytoplasm.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Cholesterol metabolism in the brain.
- Record: found
- Abstract: found
- Article: not found
Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients.
- Record: found
- Abstract: found
- Article: not found
