- Record: found
- Abstract: found
- Article: found
Frequency of nonaspirin NSAID-relevant coexisting medical conditions in the primary-care setting: a retrospective database review
Abstract
Background
Coexisting medical conditions and concomitant medications contribute to treatment challenges primary-care professionals (PCPs) face daily. The current study assessed the extent and distribution of nonaspirin NSAID-relevant coexisting medical conditions of interest (CMCOI) in patients visiting PCPs.
Methods
This retrospective database review analyzed data from three large health-care claim databases to identify the frequency of nonaspirin NSAID-relevant CMCOI among adults aged ≥18 years with a PCP visit in 2013. Claim databases employed were the Truven Health MarketScan ® Commercial Claims and Encounters database, representative of the privately insured (PI) population; Truven Health MarketScan Multi-State Medicaid, representative of the Medicaid population (Medicaid); and Truven MarketScan Medicare Supplemental, representative of the Medicare population with employer-based supplemental Medicare insurance (Medicare-Supplement). Nonaspirin NSAID-relevant CMCOI, asthma, cardiovascular risk factors, gastrointestinal bleeding risk factors, and renal insufficiency were chosen based on US NSAID over-the-counter Drug Facts label warnings. Frequency of CMCOI was determined for those without and with a musculoskeletal diagnosis.
Results
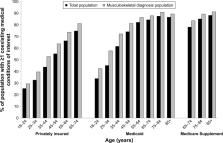
In each database, ≥19% (19.0% PI, 29.9% Medicaid, 33.6% Medicare-Supplement) had a musculoskeletal diagnosis. A greater proportion of individuals with a musculoskeletal diagnosis had one or more CMCOI compared with those without a musculoskeletal diagnosis (61.3% vs 50.4% PI, 78.1% vs 66.8% Medicaid, 87.1% vs 82.3% Medicare-Supplement). The frequency of one or more CMCOI increased with age in each database. Across databases among CMCOI, cardiovascular risk factors were most common, followed by gastrointestinal bleeding risk factors, and proportions were higher among those with a musculoskeletal diagnosis.
Conclusion
These data confirm the high frequency of nonaspirin NSAID-relevant CMCOI among patients presenting to PCPs for musculoskeletal diagnosis, as well as among older patients. These analyses reinforce the critical role health-care professionals can play in identifying patients with nonaspirin NSAID-relevant CMCOI, providing those patients with ongoing guidance on appropriate choice and use of over-the-counter analgesics, and educating patients about the impact aging, health status, concomitant conditions, and medicines have on selection of all medicines, including analgesics.
Most cited references17

- Record: found
- Abstract: found
- Article: found
Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis
- Record: found
- Abstract: not found
- Article: not found