- Record: found
- Abstract: found
- Article: not found
Quantification of the Temporal Evolution of Collagen Orientation in Mechanically Conditioned Engineered Cardiovascular Tissues
Read this article at
Abstract
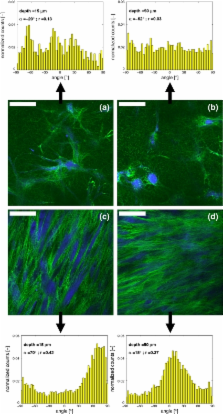
Load-bearing soft tissues predominantly consist of collagen and exhibit anisotropic, non-linear visco-elastic behavior, coupled to the organization of the collagen fibers. Mimicking native mechanical behavior forms a major goal in cardiovascular tissue engineering. Engineered tissues often lack properly organized collagen and consequently do not meet in vivo mechanical demands. To improve collagen architecture and mechanical properties, mechanical stimulation of the tissue during in vitro tissue growth is crucial. This study describes the evolution of collagen fiber orientation with culture time in engineered tissue constructs in response to mechanical loading. To achieve this, a novel technique for the quantification of collagen fiber orientation is used, based on 3D vital imaging using multiphoton microscopy combined with image analysis. The engineered tissue constructs consisted of cell-seeded biodegradable rectangular scaffolds, which were either constrained or intermittently strained in longitudinal direction. Collagen fiber orientation analyses revealed that mechanical loading induced collagen alignment. The alignment shifted from oblique at the surface of the construct towards parallel to the straining direction in deeper tissue layers. Most importantly, intermittent straining improved and accelerated the alignment of the collagen fibers, as compared to constraining the constructs. Both the method and the results are relevant to create and monitor load-bearing tissues with an organized anisotropic collagen network.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts.
- Record: found
- Abstract: found
- Article: not found
Functional living trileaflet heart valves grown in vitro.
- Record: found
- Abstract: found
- Article: not found
