- Record: found
- Abstract: found
- Article: found
A hybrid solution for extracting structured medical information from unstructured data in medical records via a double-reading/entry system
Read this article at
Abstract
Background
Healthcare providers generate a huge amount of biomedical data stored in either legacy system (paper-based) format or electronic medical records (EMR) around the world, which are collectively referred to as big biomedical data (BBD). To realize the promise of BBD for clinical use and research, it is an essential step to extract key data elements from unstructured medical records into patient-centered electronic health records with computable data elements. Our objective is to introduce a novel solution, known as a double-reading/entry system (DRESS), for extracting clinical data from unstructured medical records (MR) and creating a semi-structured electronic health record database, as well as to demonstrate its reproducibility empirically.
Methods
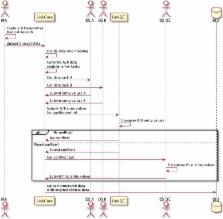
Utilizing the modern cloud-based technologies, we have developed a comprehensive system that includes multiple subsystems, from capturing MRs in clinics, to securely transferring MRs, storing and managing cloud-based MRs, to facilitating both machine learning and manual reading, and to performing iterative quality control before committing the semi-structured data into the desired database. To evaluate the reproducibility of extracted medical data elements by DRESS, we conduct a blinded reproducibility study, with 100 MRs from patients who have undergone surgical treatment of lung cancer in China. The study uses Kappa statistic to measure concordance of discrete variables, and uses correlation coefficient to measure reproducibility of continuous variables.
Results
Using the DRESS, we have demonstrated the feasibility of extracting clinical data from unstructured MRs to create semi-structured and patient-centered electronic health record database. The reproducibility study with 100 patient’s MRs has shown an overall high reproducibility of 98 %, and varies across six modules (pathology, Radio/chemo therapy, clinical examination, surgery information, medical image and general patient information).
Conclusions
DRESS uses a double-reading, double-entry, and an independent adjudication, to manually curate structured data elements from unstructured clinical data. Further, through distributed computing strategies, DRESS protects data privacy by dividing MR data into de-identified modules. Finally, through internet-based computing cloud, DRESS enables many data specialists to work in a virtual environment to achieve the necessary scale of processing thousands MRs within days. This hybrid system represents probably a workable solution to solve the big medical data challenge.
Related collections
Most cited references18
- Record: found
- Abstract: not found
- Article: not found
Biology: The big challenges of big data.
- Record: found
- Abstract: found
- Article: not found
Definition, structure, content, use and impacts of electronic health records: a review of the research literature.
- Record: found
- Abstract: found
- Article: found