- Record: found
- Abstract: found
- Article: found
Loss of calpain 3 dysregulates store-operated calcium entry and its exercise response in mice
Read this article at
Abstract
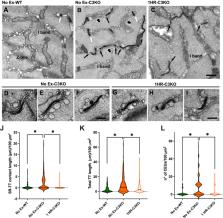
Limb-Girdle Muscular Dystrophy 2A (LGMD2A) is caused by mutations in the CAPN3 gene encoding Calpain 3, a skeletal-muscle specific, Ca 2+-dependent protease. Localization of Calpain 3 within the triad suggests it contributes to Ca 2+ homeostasis. Through live-cell Ca 2+ measurements, muscle mechanics, immunofluorescence, and electron microscopy (EM) in Capn3 deficient (C3KO) and wildtype (WT) mice, we determined if loss of Calpain 3 altered Store-Operated Calcium Entry (SOCE) activity. Direct Ca 2+ influx measurements revealed loss of Capn3 elicits elevated resting SOCE and increased resting cytosolic Ca 2+, supported by high incidence of calcium entry units (CEUs) observed by EM. C3KO and WT mice were subjected to a single bout of treadmill running to elicit SOCE. Within 1HR post-treadmill running, C3KO mice exhibited diminished force production in extensor digitorum longus muscles and a greater decay of Ca 2+ transients in flexor digitorum brevis muscle fibers during repetitive stimulation. Striking evidence for impaired exercise-induced SOCE activation in C3KO mice included poor colocalization of key SOCE proteins, stromal-interacting molecule 1 (STIM1) and ORAI1, combined with disappearance of CEUs in C3KO muscles. These results demonstrate that Calpain 3 is a key regulator of SOCE in skeletal muscle and identify SOCE dysregulation as a contributing factor to LGMD2A pathology.
Related collections
Most cited references42
- Record: found
- Abstract: not found
- Article: not found
Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A
- Record: found
- Abstract: found
- Article: not found
Orai1-dependent Calcium Entry Promotes Skeletal Muscle Growth and Limits Fatigue

- Record: found
- Abstract: found
- Article: found