- Record: found
- Abstract: found
- Article: found
The dopamine 3 receptor as a candidate biomarker and therapeutic for opioid use disorder
Read this article at
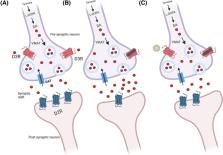
Abstract
Here, we present recent studies suggesting that specific DRD3 single nucleotide polymorphisms (SNPs, e.g. rs324029 and rs2654754) might serve as prognostic biomarkers for opioid use disorder (OUD). Additionally, preclinical studies with novel dopamine 3 receptor (D3R) partial agonists and antagonists have been evaluated as candidate OUD therapeutics and have shown a reduced risk of cardiovascular toxicity compared with the original D3R antagonist. From these findings, we argue that DRD3 SNPs could serve as a diagnostic tool for assessing OUD risk and that more research is warranted examining the D3R as a safe and effective therapeutic target for treating OUD.
Abstract
Recent studies suggest that specific DRD3 single nucleotide polymorphisms (SNPs) might serve as prognostic biomarkers for opioid use disorder (OUD). Preclinical studies with novel D3R partial agonists and antagonists have been evaluated as candidate OUD therapeutics. From these findings, we argue that DRD3 SNPs could serve as a diagnostic tool for assessing OUD risk and that more research is warranted examining the D3R as a safe and effective therapeutic target for treating OUD.
Related collections
Most cited references20
- Record: found
- Abstract: not found
- Article: not found
Comparative Effectiveness of Different Treatment Pathways for Opioid Use Disorder
- Record: found
- Abstract: found
- Article: not found
Opioids excite dopamine neurons by hyperpolarization of local interneurons.
- Record: found
- Abstract: found
- Article: not found
