- Record: found
- Abstract: found
- Article: found
Felis Catus Gammaherpesvirus 1 DNAemia in Whole Blood from Therapeutically Immunosuppressed or Retrovirus-Infected Cats
Read this article at
Abstract
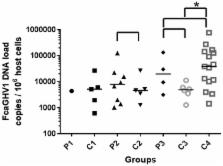
Gammaherpesviruses are major co-pathogens of human immunodeficiency virus (HIV) infection, making the interactions between feline immunodeficiency virus (FIV) and Felis catus gammaherpesvirus 1 (FcaGHV1) pertinent to both human and veterinary medical research. FIV-infected cats are at increased risk of FcaGHV1 DNAemia and consistently harbor higher FcaGHV1 loads than FIV-uninfected cats. Whether immune deficiencies unrelated to FIV are associated with similar risks is unknown. Using whole blood FcaGHV1 qPCR, we found no difference in the frequency of DNAemia or DNA load in therapeutically immunosuppressed (P1, n = 18) or feline leukemia virus (FeLV)-infected (P2, n = 57) patients compared with age- and sex-matched controls (C1, n = 58; C2, n = 57). In contrast, FIV/FeLV-co-infected cats (P3, n = 5) were at increased risk of FcaGHV1 DNAemia compared to retrovirus uninfected controls (C3, n = 39; p = 0.0068), and had a higher median FcaGHV1 DNA load, although the latter was not significant. FIV/FeLV-co-infected cats (P3) had a similar frequency of FcaGHV1 DNAemia reported compared to FIV-infected controls (C4). In conclusion, we found no evidence that cats with therapeutic immunosuppression or FeLV infection were at greater risk of FcaGHV1 DNAemia or had higher FcaGHV1 DNA load in whole blood. The risk of DNAemia in FIV/FeLV-co-infected cats was similar to that documented previously in cats infected with FIV alone.
Related collections
Most cited references37

- Record: found
- Abstract: found
- Article: found
Global burden of deaths from Epstein-Barr virus attributable malignancies 1990-2010
- Record: found
- Abstract: found
- Article: not found
Feline immunodeficiency virus (FIV) as a model for study of lentivirus infections: parallels with HIV.
- Record: found
- Abstract: found
- Article: not found