- Record: found
- Abstract: found
- Article: found
Have we achieved adequate recommendations for target volume definitions in anal cancer? A PET imaging based patterns of failure analysis in the context of established contouring guidelines
Read this article at
Abstract
Background
There are different contouring guidelines for the clinical target volume (CTV) in anal cancer (AC) which vary concerning recommendations for radiation margins in different anatomical regions, especially on inguinal site. PET imaging has become more important in primary staging of AC as a very sensitive method to detect lymph node (LN) metastases. Using PET imaging, we evaluated patterns of LN spread, and examined the differences of the respective contouring guidelines on the basis of our results.
Methods
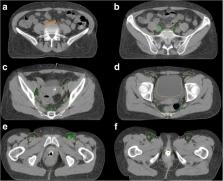
We carried out a retrospective study of thirty-seven AC patients treated with chemoradiation (CRT) who underwent FDG-PET imaging for primary staging in our department between 2011 and 2018. Patients showing PET positive LN were included in this analysis. Using a color code, LN metastases of all patients were delineated on a template with “standard anatomy” and were divided indicating whether their location was in- or out-field of the standard CTV as recommended by the Radiation Therapy Oncology Group (RTOG), the Australasian Gastrointestinal Trials Group (AGITG) or the British National Guidance (BNG). Furthermore, a detailed analysis of the location of LN of the inguinal region was performed.
Results
Twenty-two out of thirty-seven AC patients with pre-treatment PET imaging had PET positive LN metastases, accumulating to a total of 154 LN. The most commonly affected anatomical region was inguinal (49 LN, 32%). All para-rectal, external/internal iliac, and pre-sacral LN were covered by the recommended CTVs of the three different guidelines. Of forty-nine involved inguinal LN, fourteen (29%), seven (14%) and five (10%) were situated outside of the recommended CTVs by RTOG, AGITG and BNG. Inguinal LN could be located up to 5.7 cm inferiorly to the femoral saphenous junction and 2.8 cm medial or laterally to the big femoral vessels.
Conclusion
Pelvis-related, various recommendations are largely consistent, and all LN are covered by the recommended CTVs. LN “misses” appear generally cranially (common iliac or para-aortic) or caudally (inguinal) to the recommended CTVs. The established guidelines differ significantly, particular regarding the inguinal region. Based on our results, we presented our suggestions for CTV definition of the inguinal region. LN involvement of a larger number of patients should be investigated to enable final recommendations.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial.
- Record: found
- Abstract: found
- Article: not found