- Record: found
- Abstract: found
- Article: found
mmu-lncRNA 121686/hsa-lncRNA 520657 induced by METTL3 drive the progression of AKI by targeting miR-328-5p/HtrA3 signaling axis

Read this article at
Abstract
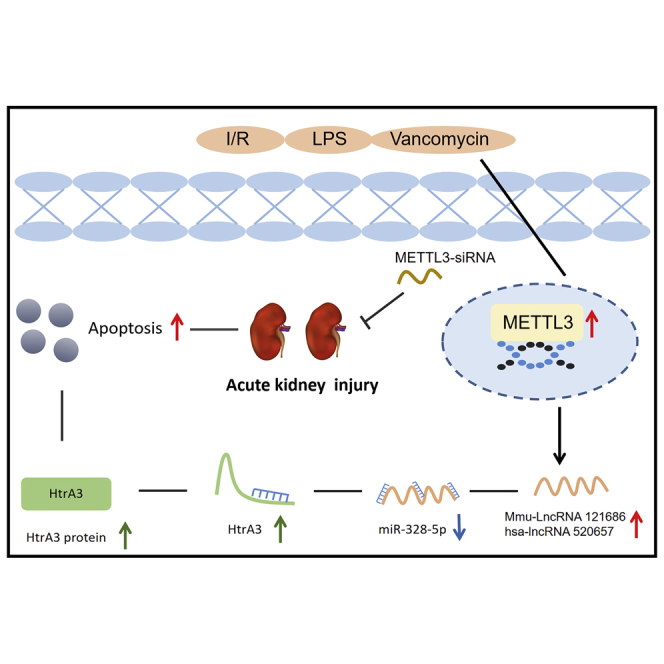
The pathogenesis of acute kidney injury (AKI) is still not fully understood, and effective interventions are lacking. Here, we explored whether methyltransferase 3 (METTL3) was involved in the progression of AKI via regulation of cell death. We reported that PT(proximal tubule)-METTL3-knockout (KO) noticeably suppressed ischemic-induced AKI via inhibition of renal cell apoptosis. Furthermore, we also found that the expression of mmu-long non-coding RNA (lncRNA) 121686 was upregulated in antimycin-treated Boston University mouse proximal tubule (BUMPT) cells and a mouse ischemia-reperfusion (I/R)-induced AKI model. Functionally, mmu-lncRNA 121686 could promote I/R-induced mouse renal cell apoptosis. Mechanistically, mmu-lncRNA 121686 acted as a competing endogenous RNA (ceRNA) to prevent microRNA miR-328-5p-mediated downregulation of high-temperature requirement factor A 3 (Htra3). PT-mmu-lncRNA 121686-KO mice significantly ameliorated the ischemic-induced AKI via the miR-328-5p/HtrA3 axis. In addition, hsa-lncRNA 520657, homologous with lncRNA 121686, sponged miR-328-5p and upregulated Htra3 to promote I/R-induced human renal cell apoptosis. Interestingly, we found that mmu-lncRNA 121686/hsa-lncRNA 520657 upregulation were dependent on METTL3 via N 6-methyladenosine (m 6A) modification. The mmu-lncRNA 121686/miR-328-5p or hsa-lncRNA 520657/miR-328-5p /HtrA3 axis was induced in vitro by METTL3 overexpression; in contrast, this effect was attenuated by METTL3 small interfering RNA (siRNA). Furthermore, we found that PT-METTL3-KO or METTL3 siRNA significantly suppressed ischemic, septic, and vancomycin-induced AKI via downregulation of the mmu-lncRNA 121686/miR-328-5p/HtrA3 axis. Taken together, our data indicate that the METTL3/mmu-lncRNA 121686/hsa-lncRNA 520657/miR-328-5p/HtrA3 axis potentially acts as a therapeutic target for AKI.
Graphical abstract
Abstract
Zhang et al. revealed that the METTL3/mmu-lncRNA 121686 or hsa-lncRNA 520657/miR-328-5p/HtrA3 axis is responsible for the progression of I/R-, CLP-, and VAN-induced AKI. Moreover, METTL3 siRNA is potential therapy way for the AKI.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
Functional Classification and Experimental Dissection of Long Noncoding RNAs
- Record: found
- Abstract: found
- Article: not found
Endogenous microRNA sponges: evidence and controversy.
- Record: found
- Abstract: found
- Article: not found