Introduction
Recent decades have seen a substantial increase in the available methods to combat diseases such as cancer and genetic disorders. Non-surgical chemotherapy is the most widely used method. However, chemotherapy not only kills tumor cells but also negatively affects normal cells and organ function. Gene therapy, whose methods are being developed as new strategies for treating cancer or genetic disorders, has gained popularity over the past few decades [1–5]. The key to the widespread clinical application of gene therapy lies in whether a gene carrier can safely transfer a gene to the desired site and enable effective expression for long time periods [6–8] to achieve lasting therapeutic effects. Nonviral cationic polymers, such as polyethyleneimine (PEI), have shown excellent application potential in gene therapy because of their non-immunogenicity, diverse structures, sizes, and potential for large-scale production [9–13]. In particular, PEI has been widely studied because of its unique “proton sponge effect.” Moreover, it is widely considered a reference standard for cationic polymer carrier performance evaluation during gene transfection [14–16].
However, the excessive positive charge on PEI/DNA polyplexes causes adverse effects, specifically high cytotoxicity, bio-incompatibility, in vivo instability, and poor cellular targeting. Negatively charged extracellular matrix components such as blood cells and plasma proteins tend to interact with polyplexes, thus decreasing their stability in body fluids and preventing their prolonged systemic circulation [17–19]. An efficient way to solve these problems is to introduce a shielding system with target groups bearing negative charges to compensate for the excess positive potential on the composite surface [20,21]. A range of materials have been developed and tested to improve the biosafety of PEI in gene therapy.
Hyaluronic acid (HA) has been recognized as a good kind of negatively charged material with outstanding potential biodegradability, and no immunogenicity or toxicity [22–24]. As a multifunctional matrix widely distributed in the human body, HA, given its unique physical and chemical properties, has many essential physiological functions, such as regulating the permeability of blood vessel walls, promoting cell growth and migration to wound sites, and regulating protein diffusion and function [25]. HA is a polysaccharide formed by alternating linkages of β-1,3-glucosaccharide or β-1,4-glucosaccharide in repeated D-glucuronic acid units and N-acetylglucosamine units [26]. The presence of functional sites, such as -COOH and -OH groups, enable easy derivatization to tailor gene encapsulation or delivery efficiency [27]. HA has been shown to target a variety of receptors, for example, cluster determinant 44 (CD44), a cell membrane glycoprotein overexpressed on tumor cells. Moreover, HA can be used for selective oncology medications, among other applications [28–30]. Using CD44 together with drugs with various modes of action can enable active targeting of drugs to tumor cells. CD44 is the primary carrier of HA on the tumor surface. Therefore, targeted drug delivery based on the interaction of HA with CD44 interaction has become the most effective and commonly used research direction in tumor-targeted drug delivery [27–32]. Shanthi et al. have engineered and screened many HA derivatives for use as shielding materials for small interfering RNA (siRNA) delivery. Complex siRNAs can be stably encapsulated by these HA derivatives, thus forming self-assembled nanosystems, which can be used to transfect RNA into tumor cells overexpressing the CD44 receptor. For example, the HA-PEI/PEG nanosystem encapsulating single-strand broken/polo-like kinase 1 (SSB/PLK1) siRNA has shown favorable efficacy and targeted specific knockout of genes in lung cancer cells (A549) [27]. Hibah et al. have demonstrated that the self-assembled nanoparticles formed by HA-coupled polyethylene glycol (HA-PEG) and HA-coupled polyethyleneimine (HA-PEI) with pDNA have high gene expression efficiency in human HeLa and A549 cell lines, and the cytotoxicity of the nanoparticles is negligible [33]. Yang et al. have designed a nanoparticle-based system composed of HA-PEI and HA-PEG, which targets CD44 receptors and delivers multidrug resistance gene 1 (MDR1) siRNA to paclitaxel-resistant OVCAR8TR tumors [34]. However, these studies were based on PEI chemical-modification strategies to obtain PEI-25k derivatives as gene carriers. The findings indicate that HA is an appropriate material for targeted gene delivery in gene therapy. PEGylation enhances cell viability and adhesion, because of PEG’s biocompatibility, as demonstrated by its applications in research on many materials [35]. To date, no studies have focused on the effects of the in vivo stability of complexes on transfection efficiency. The in vivo stability of the complex is clearly an important factor that significantly improves drug pharmacokinetics and overall efficacy. Therefore, we focused on the effects of introducing PEG to tune the material’s hydrophilic performance, thus improving the in vivo stability and prolonging the circulation of complexes for gene therapy applications.
In this work, HA or HA-PEG was used as the shielding system for pDNA/PEI polyplexes, and was found to be essential in facilitating stability, decreasing the toxicity of the complexes in vivo, and enhancing their transfection efficiency. HA-PEG was synthesized in our laboratory and used as a shielding system for a cation carrier model based on PEI-25k complexes. The negatively charged HA-PEG assembled with the positively charged complex, thereby forming a protective layer that improved the stability of the complex in vivo and suppressing the adverse effects of the PEI gene vector. Therefore, the nonspecific responses between serum proteins in vivo and the complexes decreased, and the gene transfection efficiency in vivo was improved.
Experiments
Materials
PEI-25k (25 kDa) was obtained from Sigma-Aldrich Corp. (St. Louis., MO, USA). Sodium hyaluronate (HA; 1.5–1.8 MDa) was obtained from Fluka (Czech Republic). Dulbecco’s modified Eagle medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco (Grand Island, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Amresco (Solon, Ohio, USA). Calf thymus DNA was obtained from Sigma (St. Louis, MO, USA). PEG-5k (5 kDa) was obtained from Fluka (Czech Republic).
HA-PEG synthesis
The synthesis of HA-PEG followed a protocol described previously [36]. HA sodium salt (15 mg), PEG-5k (30 mg), and 100 mg ethyl(dimethyl aminopropyl) carbodiimide were dissolved in water and mixed. The resulting solution pH was adjusted to 8.5, and the solution was then placed in an ice bath for 48 hours. The product was then dialyzed for 3 days against distilled deionized water. The HA-PEG product was freeze-dried. 1H NMR spectra of the dried product in D2O were detected with a UNit-400 NMR spectrometer.
Preparation of complexes
Equal volumes of DNA and PEI solutions were combined (w/w=1:5), mixed, and incubated for 20 min at 25 °C. Subsequently, the HA or HA-PEG solution was added dropwise into the DNA/PEI solutions and mixed to prepare the complexes with a protective layer. The rate of addition was adjusted according to the experimental results. Transfection procedures or other measurements were followed.
ζ-potential and particle size measurements
A Zeta-PALS (Brookhaven, NY) particle analyzer was used to determine the ζ-potential and particle size of the complexes (DNA/PEI/HA or DNA/PEI/HA-PEG). The complex solutions were prepared and mixed at different DNA/carrier ratios. After incubation for 20 min at 25 °C, measurements were taken.
Gel retardation assays
Agarose gel electrophoresis assays were used to detect the binding ability between the complex and the plasmid DNA (pGL3-control). The complex solutions were prepared according to the method described in Section 2.3. Solutions (10 μL) of each complex with 0.05 mg/mL pGL3-control were analyzed with 1% agarose gel electrophoresis (85 V, 1 h).
Cell culture
The cells revive in a 37 °C water bath and cultured in growth medium containing 90% high glucose DMEM, 10% fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin. Then cell culture dishes were placed in an incubator at 37 °C, with 95% humidity, and 5% CO2.
Cell viability
The cytotoxicity of the polymers was measured at 24 h. The metabolic activity of cells in each well was determined with MTT assays. The MTT solution was 5 mg/mL in PBS. In brief, in each well, 20 μL MTT solution was added. Then the plate was placed in an incubator and incubated for 4 h. The MTT solution was carefully removed, and 200 μL DMSO was added dropwise into each well to dissolve the MTT formazan crystals. The plate was incubated for an additional 10 min. Then an ELISA microplate reader (Bio-Rad) was used to record the absorbance at 492 nm for each well. The equation used to calculate cell viability (%) is as follows:

where As represents the transfected cell absorbance, and Ac represents the untransfected cell absorbance. Each measurement was repeated three times.
Free-HA competition assays
CD44 is highly expressed on the surfaces of cancer cells and is an essential HA receptor [27–32]. Flow cytometry assays were used to demonstrate the expression of the HA receptor on the surfaces of HeLa cells compared with L929 cells. HeLa cells and L929 cells were seeded into six-well plates at a density of 1.5×105 cells per well with 2 mL DMEM (containing 10% FBS) and incubated for 24 h. Then the cells in 2 mL of fresh DMEM were treated with 0.1 mL free-HA solution (1 mg/mL) for 2 h. The DNA/PEI (1/2.5), DNA/PEI/HA (1/2.5/5), and DNA/PEI/HA-PEG (1/2.5/5) complexes were then added to the plates. Finally, competition assays were performed with a BD FACSCalibur flow cytometer (BD Bioscience, USA).
Transfection
HeLa cells were transfected with the complexes (DNA/PEI, DNA/PEI/HA, or DNA/PEI/HA-PEG). First, the cells were seeded in 96-well plates (1.0×104/well) and grown in 200 μL DMEM (containing 10% FBS). Next, all cells were incubated at 37 °C for 1 day, and the transfection experiment was performed under approximately 70% confluence conditions. The medium in each well was replaced with 200 μL DMEM (containing a 15 μL complex solution made with the gel block method and 10% fetal bovine serum). The cells were further incubated with complex solutions for 2 days at 37 °C. Then a Promega luciferase analysis system was used to detect reporter gene expression. A Pierce Micro BCA Protein Assay Kit (Thermo Fisher Scientific, USA) was used to determine the protein content in the lysates. Gene transfer efficiency was measured in three replicates.
Transfection with dextran sulfate
The ternary complexes were prepared with a compound method similar to that used for the transfection experiment. Dextran sulfate (DS) at loads of 2.5, 5, 10, and 20 μg was added into the wells with 200 μL of growth medium. Plasmid DNA with a green fluorescent protein (GFP) sequence, and plasmid pGL3-control luciferase were transfected. Cells transfected with DNA with a GFP sequence were visualized under a high-power fluorescence microscope 48 h after transfection. The plasmid pGL3-control luciferase was used for quantitative detection 48 h after transfection.
Stability assays in vitro
Stability in vitro was evaluated with a nanoparticle size meter in the same manner as that used for particle size determination. The weight ratio for DNA/PEI/HA was 1/2.5/5, and the DNA/PEI/HA-PEG ratio was also 1/2.5/5. The total volume remained constant at 2 mL, and the mass percentage of NaCl was increased to the desired amount (3, 6, 9, 12, 15, 18, 21, 24, 27, and 30 mg/mL).
Pharmacokinetics
Female mice weighing 20 g were selected and fed for 1 week. Next, mouse colon cancer cells CT26 (2×106 cells/50 μL) were subcutaneously injected into the mice, and the mice continued to be fed until the tumors grew to a standard size of 0.8 cm in diameter. Biodistribution testing in vivo was then performed. The mice were randomly divided into three groups—DNA only group, DNA/PEI/HA group, and DNA/PEI/HA-PEG group—with five mice per group. Treatments of sulfo-cyanine5 (Cy5)-DNA, Cy5-DNA/PEI complex (1:2.5), Cy5-DNA/PEI/HA complex (1/2.5/5), and Cy5-DNA/PEI/HA-PEG complex (1/2.5/5) at a dose of 1 mg DNA per kg mouse body mass were administered through the tail vein. At specific time points, blood samples were collected and centrifuged at 1.0×104 rpm for 8 min. The fluorescence intensity was measured with a microplate reader (Tecan, Austria).
Biodistribution in vivo
At 6, 12, 24, and 48 h after injection, one mouse was randomly selected and sacrificed. The tumor and organs were dissected and rinsed with normal saline, immersed in 10% formaldehyde solution, and stored overnight. Luciferase imaging was performed with a Maestro in vivo Imaging System (Cambridge Research & Instrumentation, Inc., USA).
Results and discussion
HA-PEG synthesis
The structure of HA-PEG was demonstrated by 1H NMR spectra (Figure S1). The specific peaks were ascribable to PEG moieties (d 3.60 associated with -O-CH2-CH2-O-) and HA backbone (d 2.79 associated with an N-acetyl group, d 3.40–3.70 associated with glucosidic H, and d 4.70 associated with anomeric H).
Characterization of ζ-potential and particle size
Particle size analysis
As shown in Figure 1 , when the composite contained no shielding material, the particle size of the DNA/PEI complex was approximately 100 nm. With an increase in the input amount of shielding material HA or HA-PEG, the particle size of the ternary complex increased to approximately 250 nm, a size that did not influence the transfection experiments.
ζ-potential
The surface potential of the DNA/PEI complex (as shown in Figure 2 ) was +27 ±0.52 mV. In the presence of a shielding layer HA or HA-PEG, the surface potential of the complex switched from positive to negative values at a mass ratio of 1:2.5:20 and continued to increase in absolute value with increasing amounts of HA or HA-PEG. In contrast, the introduction of PEG not only improved the complex stability in solution but also decreased the negative charge of the HA surface to some extent, because of the lower charge of PEG.
Gel retardation assays
During transfection, DNA/PEI complex particles are triggered by the anionic electrolytes in the cell to release DNA. When negatively charged HA or HA-PEG is used to shield the DNA/PEI complex particles, the release of the DNA electrostatically assembled with PEI in the cell can be successfully triggered. The results demonstrated that PEI showed no DNA release when the DNA/PEI compound ratio was 1:2.5 during the Gel retardation assay, and the addition of HA or HA-PEG did not affect the combination of PEI and DNA ( Figure 3 ).

Gel retardation assay for DNA/PEI/HA (HA-PEG) ternary complex. column 1: DNA; column 2: DNA/PEI (1:2.5); column 3: DNA/PEI/HA(HA-PEG) (1:2.5:2.5); column 4: DNA/PEI/HA(HA-PEG) (1:2.5:5); column 5: DNA/PEI/HA(HA-PEG) (1:2.5:10); column 6: DNA/PEI/HA(HA-PEG) (1:2.5:20); column 7: DNA/PEI/HA(HA-PEG) (1:2.5:40); column 8: DNA/PEI/HA(HA-PEG) (1:2.5:80) and (A): HA; (B): HA-PEG.
In vitro characterization
Cell viability
The cytotoxicity of the complex systems (formed by DNA/PEI/HA or DNA/PEI/HA-PEG) was measured with MTT assays 48 h after transfection. The preparation conditions of the complexes were the same as those used during transfection. In Figure 4 , the cell viability under the complex solutions was determined under the optimized transfection conditions. The cell survival rate significantly increased with increasing input amounts of HA until the input mass ratio reached 1:2.5:5. For HA-PEG, an input mass ratio was achieved 1:2.5:20, because of compensation of the positive potential on the gene carrier surface by HA or HA-PEG, thus decreasing the cytotoxicity of the cationic carrier PEI ( Figure 2 ). These findings, combined with the surface potential data, indicated that the introduction of PEG decreased the negative charge of HA-PEG below that of HA at the same mass. Thus HA-PEG had better performance and significantly decreased the toxicity of the complex.
Flow cytometry assays
To demonstrate that the receptors of HA target only tumor cells such as HeLa cells but not normal cells such as L929 cells, we used flow cytometry assays. In Figure 5 , the most significant difference was observed in the HA or HA-PEG group, compared with the DNA/PEI group, owing to HA binding the receptors on surface of HeLa cells. However, we observed no significant differences in all groups of L929 cells. Thus, the HA receptors are overexpressed on the surfaces of HeLa cells but not L929 cells.
Transfection
The HA-PEG potential for nucleic acid delivery was assessed in vitro by transfecting HeLa cell lines with PEI-based polyplexes with plasmid luciferase DNA (pGL3-control) as the reporter gene. Ternary complexes of DNA/PEI/HA increased the transfection efficiency with increasing HA amounts ( Figure 6 ). However, the transfer efficiency decreased rapidly when the ratio of HA to PEI exceeded the 5:2.5 threshold. Meanwhile, the transfection efficiency of HA was slightly higher than that of HA-PEG under the same mass ratio until the ratio reached 1:2.5:40, because PEG obscured the negative charges on the surface layer of HA. At the high ratio of 1:2.5:80, an opposite effect on the transfection efficiency of the complex with HA or HA-PEG was observed, because the high electronegativity of HA was not conducive to endocytosis in the transfection process. Simultaneously, the slightly weaker electronegativity of HA-PEG yielded better results.
Transfection with dextran sulfate
To demonstrate that HA-PEG enhanced the stability of gene vectors in vivo, we simulated the environment in vivo by using negatively charged DS to mimic the negatively charged components in body fluids, such as the plasma and extracellular matrix. As shown in Figures 7 (A) and (B), the transfection efficiency rapidly decreased with each incremental increase in DS concentration when the complex was unshielded. When HA was used as a coating layer, the addition of DS initially resulted in a notable decrease in transfer efficiency. However, further expansion of DS did not exert any significant effect. For HA-PEG, the transfection efficiency increased rather than decreased with the amount of DS. These results indicated that HA plays an essential role in shielding the multiply charged complex surface during transfection. Simultaneously, the introduction of PEG diminished the conditioning effect of the extracellular matrix on the gene carrier in the transfection process.

(A) Qualitative transfection of ternary complexes with dextran sulfate concentrations of 0, 2.5, 5, and 10 μg. Control: Cy5-DNA only; PEI: complex of Cy5-DNA/PEI; HA: Cy5-DNA/PEI/HA complex; HA-PEG: CY5-DNA/PEI/HA-PEG complex. (B) Quantitative transfection of ternary complexes with dextran sulfate concentrations of 0, 2.5, 5, 10, and 20 μg. (C) Particle sizes of the complexes at different NaCl content.
Stability assays in vitro
In this experiment, the NaCl concentration was used to mimic the negatively charged substances in the environment in vivo. As shown in the experimental results in Figure 7 (C), with increasing NaCl concentration, the particle sizes of DNA/PEI polyplexes markedly increased, particularly at NaCl >15 mg/mL. The particle sizes of DNA/PEI/HA ternary complexes also increased with NaCl concentration. Only the particle size of the DNA/PEI/HA-PEG complex remained relatively stable, first increasing with increasing NaCl concentration and then decreasing when the concentration of NaCl was increased above 12 mg/mL. This stability was likely to be due to the conditioning effect of PEG, which helped avoid interference from negatively charged substances.
Characterization in vivo
Pharmacokinetics
The concentration of Cy5-DNA in the blood was associated with the stability of the complexes in systemic circulation in vivo through pharmacokinetics assays. In Figure 8 , the concentration of naked Cy5-DNA showed a rapid decrease after it goes into the blood circulation. The DNA/PEI showed a relatively slow decrease process. For the complexes with HA or HA-PEG, the blood systemic circulation required more time to clear the Cy5-DNA, because the shielding layer on the complex formed by HA or HA-PEG increased the stability in vivo; therefore, the HA-PEG showed better performance.
Stability and biodistribution
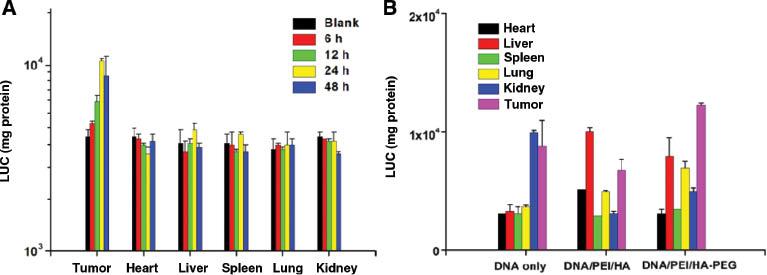
The concentration and distribution of the complex in the main organs and tumors in tumor-bearing mice were studied. At 6, 12, 24, and 48 h after the injection, one mouse injected with complexes was randomly selected and sacrificed. Major organs and tumors were studied with luciferase assays to assess the transfection efficiency. Figure 9 (A) shows the results of gene transfection with HA-PEG coating for different time points after injection. The results of gene transfection with HA-PEG coating indicated that the transfection was stable and effective in a 48-h follow-up experiment. Moreover, the transfection efficiency at the tumor site continued to improve over time, whereas other organs showed stable transfection efficiency with time. Figure 9 (B) shows the luciferase intensity of organs and tumors in mice 24 h after tail vein administration. In comparison, Figure 10 shows ex vivo images of Cy5-DNA accumulation in the main organs and tumors at 24 h. After transfection with only Cy5-DNA, the drug accumulated mainly in the kidneys and tumors at 24 h, because of the rapid metabolism in vivo. When HA or HA-PEG was introduced as a shielding material for Cy5-DNA/PEI complexes, the accumulation in the kidneys decreased, whereas the accumulation visibly increased in the liver and lungs. Notably, the transfection effect was significantly improved at tumor sites when HA-PEG was used as a shield.
Conclusion
An efficient shielding material was designed via modification of HA by water-soluble macromolecular PEG to increase the stability of the nano-complex. As a shielding material, PEGylated HA decreased the extra surface charges on the carrier/DNA complex, the cell toxicity, and the nonspecific reactions between the particles and blood cells or serum proteins. In addition to simulating the in vivo environment by using negatively charged particles in vitro, we performed in vivo biodistribution studies. HA-PEG/PEI/DNA exhibited improved stability and enhanced tumor targeting in vivo beyond those of HA/PEI/DNA and DNA. The technique described herein effectively solves the problem of excess positive charges on the surfaces of cationic gene carriers in gene transfection and thus may be highly promising for targeted delivery in gene therapy.