- Record: found
- Abstract: found
- Article: found
Oral Zinc Supplementation Reduces the Erythropoietin Responsiveness Index in Patients on Hemodialysis
Read this article at
Abstract
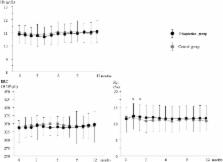
Background: In hemodialysis (HD) patients, zinc depletion caused by inadequate intake, malabsorption, and removal by HD treatment leads to erythropoiesis-stimulating agent (ESA) hyporesponsiveness. This study investigated the effects of zinc supplementation in HD patients with zinc deficiency on changes in the erythropoietin responsiveness index (ERI). Methods: Patients on HD with low serum zinc levels (<65 μg/dL) were randomly assigned to two groups: The polaprezinc group (who received daily polaprezinc, containing 34 mg/day of zinc) ( n = 35) and the control group (no supplementation) ( n = 35) for 12 months. All the 70 patients had been taking epoetin alpha as treatment for renal anemia. ERI was measured with the following equation: Weekly ESA dose (units)/dry weight (kg)/hemoglobin (g/dL). Results: There were no significant changes in hemoglobin levels within groups or between the control and polaprezinc groups during the study period. Although reticulocyte counts were increased immediately after zinc supplementation, this change was transient. Serum zinc levels were significantly increased and serum copper levels were significantly decreased in the polaprezinc group after three months; this persisted throughout the study period. Although there was no significant change in the serum iron or transferrin saturation levels in the polaprezinc group during the study period, serum ferritin levels significantly decreased following polaprezinc treatment. Further, in the polaprezinc group, ESA dosage and ERI were significantly decreased at 10 months and nine months, respectively, as compared with the baseline value. Multiple stepwise regression analysis revealed that the change in the serum zinc level was an independent predictor of lowered ERI. Conclusions: Zinc supplementation reduces ERI in patients undergoing HD and may be a novel therapeutic strategy for patients with renal anemia and low serum zinc levels.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response.
- Record: found
- Abstract: found
- Article: not found
Anemia as a risk factor for cardiovascular disease in The Atherosclerosis Risk in Communities (ARIC) study.
- Record: found
- Abstract: found
- Article: not found