- Record: found
- Abstract: found
- Article: found
Caveolin-1 and MLRs: A potential target for neuronal growth and neuroplasticity after ischemic stroke
Read this article at
Abstract
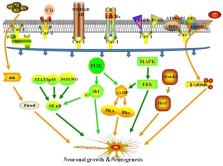
Ischemic stroke is a leading cause of morbidity and mortality worldwide. Thrombolytic therapy, the only established treatment to reduce the neurological deficits caused by ischemic stroke, is limited by time window and potential complications. Therefore, it is necessary to develop new therapeutic strategies to improve neuronal growth and neurological function following ischemic stroke. Membrane lipid rafts (MLRs) are crucial structures for neuron survival and growth signaling pathways. Caveolin-1 (Cav-1), the main scaffold protein present in MLRs, targets many neural growth proteins and promotes growth of neurons and dendrites. Targeting Cav-1 may be a promising therapeutic strategy to enhance neuroplasticity after cerebral ischemia. This review addresses the role of Cav-1 and MLRs in neuronal growth after ischemic stroke, with an emphasis on the mechanisms by which Cav-1/MLRs modulate neuroplasticity via related receptors, signaling pathways, and gene expression. We further discuss how Cav-1/MLRs may be exploited as a potential therapeutic target to restore neuroplasticity after ischemic stroke. Finally, several representative pharmacological agents known to enhance neuroplasticity are discussed in this review.
Related collections
Most cited references145
- Record: found
- Abstract: found
- Article: not found
Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth.
- Record: found
- Abstract: found
- Article: not found
Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GTPases
- Record: found
- Abstract: found
- Article: not found