- Record: found
- Abstract: found
- Article: found
A Randomised, Double Blind, Placebo-Controlled Pilot Study of Oral Artesunate Therapy for Colorectal Cancer ☆ ☆☆
Read this article at
Abstract
Background
Artesunate is an antimalarial agent with broad anti-cancer activity in in vitro and animal experiments and case reports. Artesunate has not been studied in rigorous clinical trials for anticancer effects.
Aim
To determine the anticancer effect and tolerability of oral artesunate in colorectal cancer (CRC).
Methods
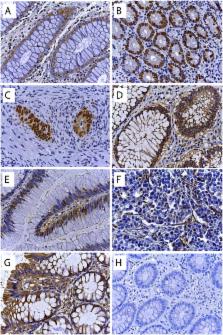
This was a single centre, randomised, double-blind, placebo-controlled trial. Patients planned for curative resection of biopsy confirmed single primary site CRC were randomised (n = 23) by computer-generated code supplied in opaque envelopes to receive preoperatively either 14 daily doses of oral artesunate (200 mg; n = 12) or placebo (n = 11). The primary outcome measure was the proportion of tumour cells undergoing apoptosis (significant if > 7% showed Tunel staining). Secondary immunohistochemical outcomes assessed these tumour markers: VEGF, EGFR, c-MYC, CD31, Ki67 and p53, and clinical responses.
Findings
20 patients (artesunate = 9, placebo = 11) completed the trial per protocol. Randomization groups were comparable clinically and for tumour characteristics. Apoptosis in > 7% of cells was seen in 67% and 55% of patients in artesunate and placebo groups, respectively. Using Bayesian analysis, the probabilities of an artesunate treatment effect reducing Ki67 and increasing CD31 expression were 0.89 and 0.79, respectively. During a median follow up of 42 months 1 patient in the artesunate and 6 patients in the placebo group developed recurrent CRC.
Highlights
-
•
Artesunate is a cheap and orally available, widely used antimalarial drug that has anticancer properties in in vitro models
-
•
Tested the effects of artesunate on colorectal cancer in patients before they have had surgery to remove the tumour
-
•
Results from this pilot study are promising and support further studies to explore anticancer effects of artemisinins
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Pre-referral rectal artesunate to prevent death and disability in severe malaria: a placebo-controlled trial
- Record: found
- Abstract: found
- Article: not found
Caspase control: protagonists of cancer cell apoptosis.
- Record: found
- Abstract: found
- Article: not found