- Record: found
- Abstract: found
- Article: found
Effects of common cold and concomitant administration of nasal decongestant on the pharmacokinetics and pharmacodynamics of nasal glucagon in otherwise healthy participants: A randomized clinical trial
Read this article at
Abstract
Aims
Nasal glucagon (NG) is a nasally‐administered glucagon powder, absorbed through the nasal mucosa, designed for treatment of severe hypoglycaemia. This study evaluated the safety, pharmacokinetics (PK) and pharmacodynamics (PD) of NG in otherwise healthy participants with common colds and after recovery from cold symptoms, with and without concomitant nasal decongestant.
Materials and Methods
This was a single‐centre, open‐label study. Cohort 1 participants (N = 18) received 2 doses of NG: one while experiencing nasal congestion and another after recovery from cold symptoms. Cohort 2 participants (N = 18), who also had colds with nasal congestion, received a single dose of NG 2 hours after treatment with the decongestant oxymetazoline. Total symptoms score and other safety measures were assessed before and after NG administration.
Results
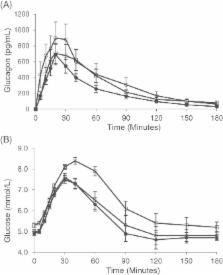
NG was well tolerated, without serious adverse events. Common adverse events (transient lacrimation, nasal discomfort, rhinorrhea and nausea) were more frequent in both Cohorts 1 and 2 during nasal congestion. Glucagon levels peaked 18 minutes post‐dose and glucose levels peaked 30 to 42 minutes post‐dose in all groups. Nasal congestion, with or without concomitant nasal decongestant, did not significantly affect PK of NG. Although glucose AUECs 0‐t was different between Cohort 1 with nasal congestion and Cohort 2, glucose concentrations at 30 minutes appeared similar in all groups.
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: not found
Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry.
- Record: found
- Abstract: found
- Article: not found
Intranasal drug delivery: how, why and what for?
- Record: found
- Abstract: found
- Article: not found