- Record: found
- Abstract: found
- Article: found
Epstein Barr Virus Associated Lymphomas and Epithelia Cancers in Humans
Read this article at
Abstract
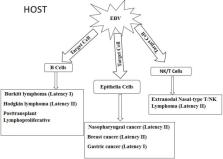
Epstein Barr virus (EBV) is a cosmopolitan oncogenic virus, infecting about 90% of the world's population and it is associated to tumors originating from both epithelia and hematopoietic cells. Transmission of the virus is mainly through oral secretions; however, transmission through organ transplantation and blood transfusion has been reported. In order to evade immune recognition, EBV establishes latent infection in B lymphocytes where it expresses limited sets of proteins called EBV transcription programs (ETPs), including six nuclear antigens (EBNAs), three latent membrane proteins (LMP), and untranslated RNA called EBV encoded RNA (EBER), shown to efficiently transform B cells into lymphoblastic cells. These programs undergo different patterns of expression which determine the occurrence of distinct types of latency in the pathogenesis of a particular tumor. Hematopoietic cell derived tumors include but not limited to Burkitt's lymphoma, Hodgkin lymphoma, post-transplant lymphoproliferative disorders, and natural killer (NK)/T cell lymphoma. EBV undergoes lytic infection in epithelia cells for amplification of the viral particle for transmission where it expresses lytic stage genes. However, for reasons yet to be unveiled, EBV switches from the expression of lytic stage genes to the expression of ETPs in epithelia cells. The expression of the ETPs lead to the transformation of epithelia cells into permanently proliferating cells, resulting in epithelia cell derived malignancies such as nasopharyngeal cancer, gastric cancer, and breast cancer. In this review, we have summarized the current updates on EBV associated epithelial and B cell-derived malignancies, and the role of EBV latency gene products in the pathogenesis of the cancers, and have suggested areas for future studies when considering therapeutic measures
Related collections
Most cited references146
- Record: found
- Abstract: found
- Article: not found
Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA.
- Record: found
- Abstract: found
- Article: not found
A biologic definition of Burkitt's lymphoma from transcriptional and genomic profiling.
- Record: found
- Abstract: found
- Article: not found