- Record: found
- Abstract: found
- Article: found
The new paradigm of hepatitis C therapy: integration of oral therapies into best practices
Read this article at
Abstract
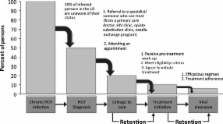
SUMMARY. Emerging data indicate that all-oral antiviral treatments for chronic hepatitis C virus (HCV) will become a reality in the near future. In replacing interferon-based therapies, all-oral regimens are expected to be more tolerable, more effective, shorter in duration and simpler to administer. Coinciding with new treatment options are novel methodologies for disease screening and staging, which create the possibility of more timely care and treatment. Assessments of histologic damage typically are performed using liver biopsy, yet noninvasive assessments of histologic damage have become the norm in some European countries and are becoming more widespread in the United States. Also in place are new Centers for Disease Control and Prevention (CDC) initiatives to simplify testing, improve provider and patient awareness and expand recommendations for HCV screening beyond risk-based strategies. Issued in 2012, the CDC recommendations aim to increase HCV testing among those with the greatest HCV burden in the United States by recommending one-time testing for all persons born during 1945–1965. In 2013, the United States Preventive Services Task Force adopted similar recommendations for risk-based and birth-cohort-based testing. Taken together, the developments in screening, diagnosis and treatment will likely increase demand for therapy and stimulate a shift in delivery of care related to chronic HCV, with increased involvement of primary care and infectious disease specialists. Yet even in this new era of therapy, barriers to curing patients of HCV will exist. Overcoming such barriers will require novel, integrative strategies and investment of resources at local, regional and national levels.
Related collections
Most cited references107
- Record: found
- Abstract: not found
- Article: not found
Diagnosis, management, and treatment of hepatitis C: an update.
- Record: found
- Abstract: found
- Article: not found
Boceprevir for untreated chronic HCV genotype 1 infection.
- Record: found
- Abstract: found
- Article: not found