- Record: found
- Abstract: found
- Article: not found
ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain) – a second-generation smallpox vaccine for biological defense
Read this article at
Summary
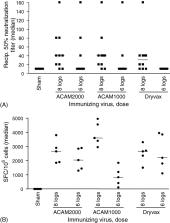
The threat of smallpox as a biological weapon has spurred efforts to create stockpiles of vaccine for emergency preparedness. In lieu of preparing vaccine in animal skin (the original method), we cloned vaccinia virus (New York City Board of Health strain, Dryvax ®) by plaque purification and amplified the clone in cell culture. The overarching goal was to produce a modern vaccine that was equivalent to the currently licensed Dryvax ® in its preclinical and clinical properties, and could thus reliably protect humans against smallpox. A variety of clones were evaluated, and many were unacceptably virulent in animal models. One clonal virus (ACAM1000) was selected and produced at clinical grade in MRC-5 human diploid cells. ACAM1000 was comparable to Dryvax ® in immunogenicity and protective activity but was less neurovirulent for mice and nonhuman primates. To meet requirements for large quantities of vaccine after the events of September 11th 2001, the ACAM1000 master virus seed was used to prepare vaccine (designated ACAM2000) at large scale in Vero cells under serum-free conditions. The genomes of ACAM1000 and ACAM2000 had identical nucleotide sequences, and the vaccines had comparable biological phenotypes. ACAM1000 and ACAM2000 were evaluated in three Phase 1 clinical trials. The vaccines produced major cutaneous reactions and evoked neutralizing antibody and cell-mediated immune responses in the vast majority of subjects and had a reactogenicity profile similar to that of Dryvax ®.
Related collections
Most cited references24
- Record: found
- Abstract: not found
- Article: not found
Complications of smallpox vaccination, 1968.
- Record: found
- Abstract: found
- Article: not found
Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination.
- Record: found
- Abstract: found
- Article: not found
