- Record: found
- Abstract: found
- Article: found
RPS15A promotes gastric cancer progression via activation of the Akt/IKK‐β/NF‐κB signalling pathway
Read this article at
Abstract
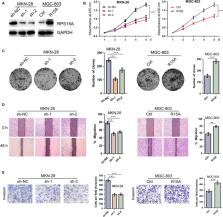
This study aimed to investigate the clinical significance, potential biological function and underlying mechanism of RPS15A in gastric cancer (GC) progression. RPS15A expression was detected in 40 pairs of GC tissues and matched normal gastric mucosae (MNGM) using qRT‐PCR analysis. Immunohistochemistry assay was conducted using a tissue microarray including 186 primary GC samples to characterize the clinical significance of RPS15A. A series of in vitro and in vivo assays were performed to elucidate the biological function of RPS15A in GC development and underlying molecular mechanisms. The expression of RPS15A was significantly up‐regulated in GC samples compared to MNGM, and its expression was closely related to TNM stage, tumour size, differentiation, lymph node metastasis and poor patient survival. Ectopic expression of RPS15A markedly enhanced the proliferation and metastasis of GC cells both in vitro and in vivo. RPS15A overexpression also promoted the epithelial‐mesenchymal transition (EMT) phenotype formation of GC cells. Investigations of underlying mechanisms found that RPS15A activated the NF‐κB signalling pathway by inducing the nuclear translocation and phosphorylation of the p65 NF‐κB subunit, transactivation of NF‐κB reporter and up‐regulating target genes of this pathway. In addition, RPS15A overexpression activated, while RPS15A knockdown inhibited the Akt/IKK‐β signalling axis in GC cells. And both Akt inhibitor LY294002 and IKK inhibitor Bay117082 neutralized the p65 and p‐p65 nuclear translocation induced by RPS15A overexpression. Collectively, our findings suggest that RPS15A activates the NF‐κB pathway through Akt/IKK‐β signalling axis, and consequently promotes EMT and GC metastasis. This newly identified RPS15A/Akt/IKK‐β/NF‐κB signalling pathway may be a potential therapeutic target to prevent GC progression.
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: not found
Hierarchies of NF-κB target-gene regulation.
- Record: found
- Abstract: found
- Article: not found
IGFBP2 Activates the NF-κB Pathway to Drive Epithelial-Mesenchymal Transition and Invasive Character in Pancreatic Ductal Adenocarcinoma.
- Record: found
- Abstract: found
- Article: not found