- Record: found
- Abstract: found
- Article: found
An overview of vaccine development for COVID-19
Read this article at
Abstract
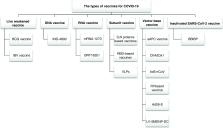
The COVID-19 pandemic continues to endanger world health and the economy. The causative SARS-CoV-2 coronavirus has a unique replication system. The end point of the COVID-19 pandemic is either herd immunity or widespread availability of an effective vaccine. Multiple candidate vaccines – peptide, virus-like particle, viral vectors (replicating and nonreplicating), nucleic acids (DNA or RNA), live attenuated virus, recombinant designed proteins and inactivated virus – are presently under various stages of expansion, and a small number of vaccine candidates have progressed into clinical phases. At the time of writing, three major pharmaceutical companies, namely Pfizer and Moderna, have their vaccines under mass production and administered to the public. This review aims to investigate the most critical vaccines developed for COVID-19 to date.
Related collections
Most cited references69
- Record: found
- Abstract: found
- Article: not found
Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine
- Record: found
- Abstract: found
- Article: not found
Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine

- Record: found
- Abstract: found
- Article: found