- Record: found
- Abstract: found
- Article: found
Pharmacokinetic Characteristics, Pharmacodynamic Effect and In Vivo Antiviral Efficacy of Liver-Targeted Interferon Alpha
Read this article at
Abstract
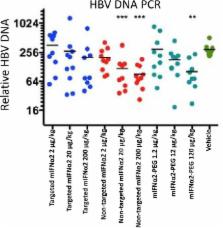
Interferon alpha (IFNα) is used for the treatment of hepatitis B virus infection, and whilst efficacious, it is associated with multiple adverse events caused by systemic exposure to interferon. We therefore hypothesise that targeting IFN directly to the intended site of action in the liver would reduce exposure in blood and peripheral tissue and hence improve the safety and tolerability of IFNα therapy. Furthermore we investigated whether directing IFN to the reservoir of infection in the liver may improve antiviral efficacy by increasing local concentration in target organs and tissues. Our previous results show that the mIFNα2 fused to an ASGPR specific liver targeting antibody, DOM26h-196-61, results in a fusion protein which retains the activity of both fusion partners when measured in vitro. In vivo targeting of the liver by mIFNα2-DOM26h-196-61, hereafter referred to as targeted mIFNα2, was observed in microSPECT imaging studies in mice. In this study we show by pharmacokinetic analysis that antibody mediated liver-targeting results in increased uptake and exposure of targeted mIFNα2 in target tissues, and correspondingly reduced uptake and exposure in systemic circulation, clearance organs and non-target tissues. We also show that cytokine activity and antiviral activity of liver-targeted IFN is observed in vivo, but that, contrary to expectations, liver-targeting of mIFNα2 using ASGPR specific dAbs actually leads to a reduced pharmacodynamic effect in target organs and lower antiviral activity in vivo when compared to non-targeted mIFNα2-dAb fusions.
Related collections
Most cited references20
- Record: found
- Abstract: found
- Article: not found
Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli.
- Record: found
- Abstract: found
- Article: not found
Domain antibodies: proteins for therapy.
- Record: found
- Abstract: found
- Article: not found