- Record: found
- Abstract: found
- Article: found
Influence of insulin and glargine on outgrowth and number of circulating endothelial progenitor cells in type 2 diabetes patients: a partially double-blind, randomized, three-arm unicenter study
Read this article at
Abstract
Background
Endothelial progenitor cells (EPC) are bone marrow-derived cells which can undergo differentiation into endothelial cells and participate in endothelial repair and angiogenesis. Insulin facilitates this in vitro mediated by the IGF-1 receptor. Clinical trials showed that the number of circulating EPCs is influenced by glucose control and EPC are a predictor of cardiovascular death. To study direct effects of insulin treatment on EPCs in type 2 diabetes patients, add-on basal insulin treatment was compared to an escalation of oral medication aiming at similar glucose control between the groups.
Methods
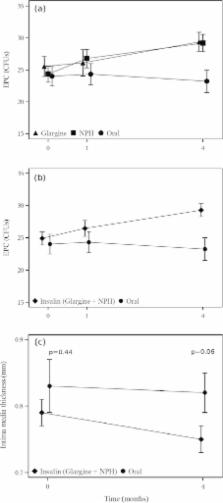
55 patients with type 2 diabetes (61.6±5.9 years) on oral diabetes medication were randomized in a 2:2:1 ratio in 3 groups. Patients were treated additionally with insulin glargine (n=20), NPH insulin (n=22) or escalated with oral medication (n=13). Number of circulating EPC, EPC-outgrowth, intima media thickness, skin microvascular function and HbA1c were documented at baseline and/or after 4 weeks and 4 months.
Results
HbA1c at baseline was, 7.3+/−0.7% in the oral group, 7.3+/−0.9% and 7.5+/−0.7% in the glargine and NPH insulin respectively (p=0.713). HbA1c after 4 months decreased to 6.8+/−0.8%, 6.6+/−0.7% and 6.7+/−0.6%, in the oral, glargine and NPH insulin group respectively (p=0.61). FACS analysis showed no difference in number of circulating EPC between the groups after 4 weeks and 4 months. However, the outgrowth of EPCs as detected by colony forming assay was increased in the NPH insulin and glargine groups (29.2+/−6.4 and 29.4+/− 6.7 units respectively) compared to the group on oral medication (23.2+/−6.3, p=0.013) after 4 months of treatment. A significant decrease of IMT from 0.80mm (+/−0.14) at baseline to 0.76mm (+/−0.12) after 4 months could be observed in all patients only (p=0.03) with a trend towards a reduction of IMT after 4 months when all patients on insulin treatment were compared to the oral treatment group (p=0.06). Skin microvascular function revealed no differences between the groups (p=0.74).
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus.
- Record: found
- Abstract: found
- Article: not found
Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group.
- Record: found
- Abstract: found
- Article: not found
