- Record: found
- Abstract: found
- Article: found
Interfacial rheology of sodium caseinate/high acyl gellan gum complexes: Stabilizing oil-in-water emulsions
Read this article at
Abstract
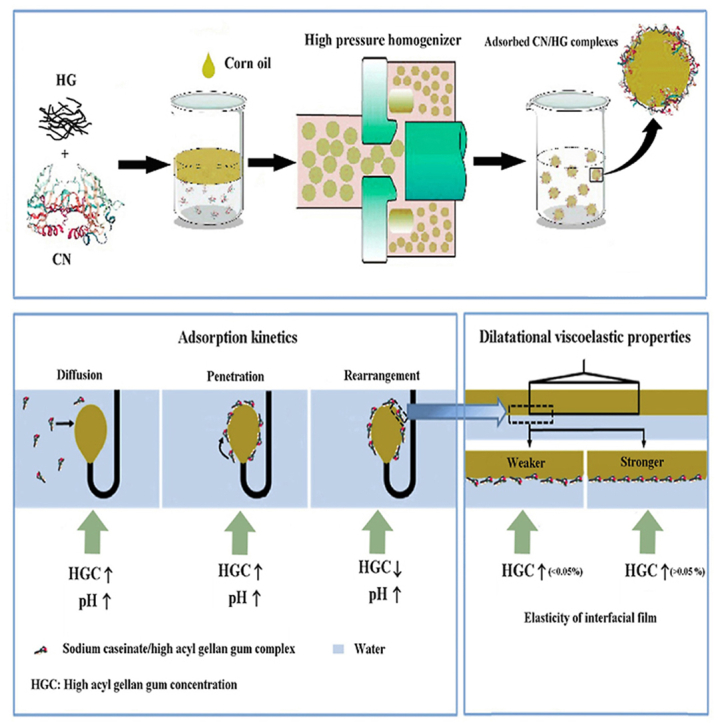
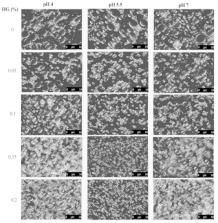
In this work, the effects of pH and high acyl gellan gum concentration on the adsorption kinetics and interfacial dilatational rheology of sodium caseinate/high acyl gellan gum (CN/HG) complexes were investigated using a pendant drop tensiometer. In addition, stability related properties including interfacial protein concentration, droplet charge, size, microstructure and creaming index of emulsions were studied at different HG concentration (0–0.2 wt%) and pH values (4, 5.5 and 7). The results showed that HG adsorbed onto the CN mainly through electrostatic interactions which could lead to increase the interfacial pressure (π), rates of protein diffusion (k diff), and molecular penetration (k p). The CN/HG complexes formed thick adsorption layers around the oil droplets which significantly increased the surface dilatational modulus with the increasing HG concentration. The CN/HG complexes appeared to form more elastic interfacial films after a long-term adsorption time compared with CN alone, which could reduce the droplet coalescence and thus prevented the growth of emulsion droplets. All four phosphorylated proteins of CN (α s1-, α s2-, β-, and κ-casein) were adsorbed at the oil-water (O/W) interface as confirmed by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), and surface protein coverage increased progressively with increasing HG concentration at pH 5.5, but decreased at pH 7. The CN/HG stabilized emulsions at pH 5.5 revealed the higher net charges and smaller z-average diameters than those at pH 4 and pH 7. This study provides valuable information on the use of CN/HG complexes to improve the stability and texture of food emulsions.
Graphical abstract
Highlights
-
•Interactions between sodium caseinate (CN) and high acyl gellan gum (HG) studied.
-
•At pH 5.5, CN/HG interaction was mainly driven by electrostatic attractions.
-
•CN/HG complex improved the adsorption of CN at the oil-water interface.
-
•CN/HG complex could form stronger interfacial films than protein alone.
-
•Cooperative adsorption onto oil-water interface improved emulsion stability.
Related collections
Most cited references46
- Record: found
- Abstract: not found
- Article: not found
Hydrocolloids at interfaces and the influence on the properties of dispersed systems
- Record: found
- Abstract: found
- Article: not found
Food proteins: a review on their emulsifying properties using a structure-function approach.
- Record: found
- Abstract: not found
- Article: not found