- Record: found
- Abstract: found
- Article: found
Local Sleep Oscillations: Implications for Memory Consolidation
discussion
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Introduction
Accumulating evidence suggests that sleep is important for plasticity and memory consolidation
(Maquet, 2001; Walker and Stickgold, 2004; Datta and Maclean, 2007; Diekelmann and
Born, 2010; Tononi and Cirelli, 2014; Dudai et al., 2015)—the transformation of new
labile memories encoded in wakefulness into stable representations that integrate
into long-term memory networks. A central model accounting for memory consolidation
during sleep is that of coupling between hippocampal (HC) and neocortical networks
(Buzsáki, 1996). According to this two-stage model of memory formation [also termed
the hippocampal—neocortical dialogue model (Buzsáki, 1989)], the dominant direction
of information flow across the brain differs between wake and sleep periods. During
wakefulness, acquisition of sensory information mainly drives signal propagation from
cortex to hippocampus (HC) (Buzsáki, 1998; Mormann et al., 2008). By contrast, during
subsequent non-rapid eye movement (NREM) sleep, this model suggests a central role
for information flow from HC to cortex especially around sharp-wave ripples (SWRs)
events (Buzsáki, 1998). Accordingly, slow waves that originate in the neocortex repeatedly
reactivate the newly encoded HC information when SWRs occur, driving subsequent activity
in select cortical circuits (Siapas and Wilson, 1998). However, it is clear that information
flow is not strictly unidirectional (Wagner et al., 2010) and may involve complex
loops (Rothschild et al., 2017). HC reactivation tends to co-occur with sleep spindles
that optimize plasticity (Seibt et al., 2017), resulting in long-term modification
of synaptic efficacy. Thus, hippocampal–neocortical coupling requires interregional
cross-frequency coordination between sleep oscillations, including slow waves and
sleep spindles in thalamo-cortical circuits as well as HC ripples.
The underlying prevalent assumption is that sleep oscillations (slow waves in particular)
are global events that co-occur nearly simultaneously across different brain regions.
But in fact, they have been described as traveling waves propagating from anterior-to-posterior
cortex (Massimini et al., 2004), and they typically occur out of phase across different
cortical sites (Nir et al., 2011; Vyazovskiy et al., 2011; Malerba et al., 2019).
How can we reconcile models requiring co-occurrence of sleep oscillations with accumulating
evidence of non-uniform timing of oscillations across the brain? In this article,
we first review the current data that sheds light on this question, and highlight
recent studies that link regional coupling of sleep oscillations with consolidation
of specific memories. Then, we highlight the gap between sleep and memory theory and
experimental evidence. Based on studies that monitor and manipulate specific cortical
circuits, we propose that coupling can occur between sleep oscillations in general,
and between HC and cortex specifically, but that such coupling likely involves different
brain regions at each point in time, contributing to memory consolidation in select
circuits.
Slow Waves, Spindles, and Their Regional Modulation Following Learning
Slow waves and sleep spindles constitute electroencephalographic (EEG) hallmarks of
NREM sleep (Gibbs and Gibbs, 1950; Steriade, 2003). These robust oscillations are
easily identified using non-invasive EEG and form the main criterion for sleep stage
definition across mammalian species (Iber et al., 2007). Both oscillations are implicated
in memory consolidation as we review below. While EEG represents summed activity across
large cortical territories (Nunez, 1995), we will focus here on accumulating evidence
that characterizes slow waves and spindles as local phenomena.
Neocortical slow waves reflect slow (1–4 Hz) alternations of cellular active (up-)
and inactive (down-) states of neuronal activity (Steriade et al., 2001; Nir et al.,
2011). Although not perfectly coherent, these oscillations represent the most synchronous
event in the healthy brain, and traveling waves across large cortical territories
may mediate diverse sleep functions including downregulation of synaptic strengths
(Vyazovskiy et al., 2008; Norimoto et al., 2018), maintenance of cellular homeostasis
(Tononi and Cirelli, 2014), and mediation of memory consolidation and synaptic plasticity
(Diekelmann and Born, 2010).
Slow waves are thought to provide a temporal frame for a dialogue between the neocortex
and subcortical structures, which is necessary for redistributing memories for long-term
storage (Sirota et al., 2003; Sirota and Buzsáki, 2005; Marshall and Born, 2007):
On a global scale, a strong increase in EEG coherence is observed during NREM sleep
following learning in humans (Mölle et al., 2004, 2009). On a local scale, changes
in sleep oscillations occur in specific cortical regions that were involved in encoding,
both in rodents (Vyazovskiy et al., 2000; Hanlon et al., 2009) and in humans (Huber
et al., 2004, 2006; Mölle et al., 2009). Although very commonly regarded as a global
event occurring near-simultaneously across the cortex, cortical up-states are typically
ignited locally in prefrontal cortex and spread to other cortical areas over tens
to a few 100 ms (Massimini et al., 2004). Neural recordings in rodents were able to
pinpoint the ignition source to layer 5 cells of cortex (Luczak et al., 2007; Chauvette
et al., 2010; Beltramo et al., 2013). Intracranial recordings from epilepsy patients
reveal that most slow waves, and the underlying active and inactive neuronal states,
occur locally (Nir et al., 2011). This observation goes beyond potential confounds
of epilepsy, since it is readily observed also in rodents and in cats (Chauvette et
al., 2011; Vyazovskiy et al., 2011). Especially during late sleep, circumscribed slow
waves are also detected via EEG recordings (Siclari et al., 2014; Bernardi et al.,
2018).
Sleep spindles are classically defined as waxing-and-waning 10–16 Hz oscillations
lasting 0.5–2 s (Gibbs and Gibbs, 1950). Sleep spindles are implicated in plasticity
and trigger synaptic long-term potentiation via calcium transients that are believed
to prime cortical networks for the long-term storage of memory representations (Timofeev
et al., 2002; Rosanova and Ulrich, 2005; Ulrich, 2016; Niethard et al., 2018). On
a global scale, increased spindle activity is observed during NREM sleep following
learning of both declarative tasks and procedural motor skills (Gais et al., 2002;
Eschenko et al., 2006; Fogel and Smith, 2006; Morin et al., 2008; Mölle et al., 2009).
On a local scale, regional spindle activity correlates with offline improvement in
consolidation of motor memories (Nishida and Walker, 2007). Importantly, despite the
fact that spindles engage thalamo-cortical “loops,” they are also mostly a local phenomenon
occurring in select circuits at a time (Rasch and Born, 2013). Even when observed
near-simultaneously across regions, their precise timings varies across cortical locations
(Nir et al., 2011; Muller et al., 2016). Accordingly, learning different types of
memories changes the properties of spindles in different topographically-restricted
regions (Bergmann et al., 2012; Cox et al., 2014).
Not only are slow waves and sleep spindles each related to memory consolidation separately,
recent evidence suggests that their precise interaction may play a role. For example,
many sleep spindles tend to be “nested” in the “up” phase of the slow oscillation
as revealed by phase-amplitude coupling (PAC) analysis (Diekelmann and Born, 2010;
Staresina et al., 2015). However, slow wave and spindle oscillations behave as traveling
waves at a whole-brain scale [for an extensive review see (Muller et al., 2018)],
which translates to a delay of up to hundreds of milliseconds between oscillation
peaks across different cortical areas. Thus, the temporal relationship between sleep
oscillations across cortical regions varies substantially. Locally, within each brain
region, the coupling of sleep spindles to slow wave up-states occurs in a topographically
restricted fashion (Cox et al., 2014) and local slow waves coordinate spindle activity
at virtually every cortical site (Cox et al., 2018). In contrast, coupling between
distant brain regions does not necessarily occur regularly. For example, while parietal
spindles are coupled to parietal slow waves, they are not necessarily coupled with
frontal slow waves (Figure 1). Along this line, the strength of slow wave-spindle
coupling differs between global and local slow waves, as well as between cortical
locations (Malerba et al., 2019), highlighting the complexity of cross-frequency coupling
between sleep oscillations across different brain regions.
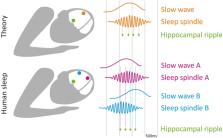
Figure 1
Local sleep oscillations and memory consolidation: theory vs. experimental findings.
(Top) Theory suggests that the nesting of hippocampal ripples (green) to sleep spindle
troughs (orange), which in turn are nested in slow wave up-phase (orange), is critical
for memory consolidation during sleep. (Bottom) Experimental data indicates that timing
of both slow waves and spindles (as well as spindle nesting phase; Andrillon et al.,
2011) varies across cortical regions (purple and blue), such that the nesting of each
ripple (green) inevitably corresponds to different cortical locations (dashed vertical
lines). Thus, each hippocampal ripple occurring at a specific time is associated with
hippocampal-cortical coupling in different circuits, likely supporting memory consolidation
related to that circuit.
Interregional Coupling Between Hippocampus and Specific Cortical Regions During Sleep,
and Its Role in Successful Memory Consolidation
During NREM sleep, hippocampal (HC) activity is concentrated in sharp wave ripple
(SWR) events, which correspond to a summed synchronous depolarization of a large fraction
of the neurons in the CA1 sub region of the hippocampus (O'keefe and Nadel, 1978;
Buzsáki et al., 1983; Buzsáki, 1986). Extensive animal research established a tight
link between HC SWRs and memory consolidation in both wakefulness and sleep: SWRs
accompany the sleep-associated re-activation of HC neuron ensembles that were active
during the preceding awake learning experience (Nadasdy et al., 1999; Eschenko et
al., 2008; Peyrache et al., 2009). SWRs occurrence increases in previously potentiated
synaptic circuits (Behrens et al., 2005), and may further modulate synaptic strength
(Buzsáki et al., 1987; King et al., 1999; Norimoto et al., 2018). Finally, selective
manipulation of SWRs through electrical or optogenetic stimulation in HC modulates
memory consolidation (Girardeau et al., 2009; Ego-Stengel and Wilson, 2010; Fernandez-Ruiz
et al., 2019). Thus, SWRs represent important time epochs for offline HC activity,
and their occurrence in NREM sleep carries a privileged role in plasticity and memory
consolidation.
In deep layers of medial prefrontal cortex (mPFC), where most of the HC fibers make
contacts, pyramidal cells respond phasically to SWRs (Siapas and Wilson, 1998; Mölle
et al., 2006; Peyrache et al., 2011). Conversely, the occurrence of SWRs is modulated
by neocortical inputs (Isomura et al., 2006), revealing bidirectional interactions
between HC and cortex. Multiple studies revealed the fine temporal relationship between
SWRs and neocortical sleep oscillations (Sirota et al., 2003; Sirota and Buzsáki,
2005; Staresina et al., 2015; Wang and Ikemoto, 2016), in which SWRs tend to be phase-locked
to cortical spindle troughs, which in turn are phase-locked to slow wave up-states.
Human studies are typically limited in SWR detection, as non-invasive EEG cannot reliably
monitor local high-frequency activities in deep brain structures. Nevertheless, sleep
studies in epilepsy patients implanted with intracranial electrodes support the notion
that SWRs during sleep preferentially occur at specific times in relation to neocortical
slow waves and spindles (Clemens et al., 2007, 2011; Nir et al., 2011; Staresina et
al., 2015), extending the temporal tuning finding from rodents to human sleep. Given
that spindles are mostly a local phenomenon, and their precise timing varies across
cortical locations (Nir et al., 2011; Muller et al., 2016), temporal tuning between
one cortical area and HC during a specific spindle does not necessarily imply temporal
tuning between other cortical areas to HC at that time (Figure 1).
At present, a gap exists between theory on how hippocampal-cortical coupling supports
memory consolidation (usually considering the entire cortex as a uniform entity) and
the available experimental evidence highlighting that slow waves, spindles, and SWRs
occur at different times in different regions.
Coupling of Sleep Oscillations in Select Brain Regions and the Consolidation of Specific
Memories
A potential way to transcend this discrepancy is to consider that coupling between
sleep oscillations may occur, but may involve select circuits at each given time—supporting
memory consolidation in specific associated tasks. We illustrate this by considering
two recent studies in rodents that causally link the coupling of sleep oscillations
across specific regions to the consolidation of specific memories. A recent study
(Maingret et al., 2016) established that co-occurrence of HC ripples and medial prefrontal
cortex (mPFC) slow waves and spindles correlates with memory consolidation in a spatial
learning task. Boosting this coupling by delivering SWR-triggered electrical stimulation
to deep cortical layers causally improved memory performance on this hippocampus-dependent
task (Maingret et al., 2016). Another study used a different closed-loop stimulation
protocol to improve memory performance in a hippocampal dependent task: frontal slow
waves triggered optogenetic stimulation of the thalamic reticular nucleus during sleep,
resulting in time-locked frontal sleep spindles, and HC SWRs (Latchoumane et al.,
2017). Notably, these experiments, as well as studies selectively manipulating SWRs,
report changes in coupling between SWRs in a specific hippocampal (HC) sub-field [mostly
CA1 (Girardeau et al., 2009; Ego-Stengel and Wilson, 2010; Maingret et al., 2016)],
and spindles in specific regions [either mPFC (Siapas and Wilson, 1998) or anterior
cingulate cortex (Wang and Ikemoto, 2016)]. Thus, these findings demonstrate that
although each SWR may be coupled with slow waves and spindle oscillations in different
brain regions (Figure 1), HC-cortical coupling in select circuits may support memory
consolidation in specific tasks.
Although the majority of sleep and memory experiments focus on temporal coupling between
HC and cortex, several studies also demonstrate the importance of coherence between
specific cortical regions. Miyamoto and colleagues demonstrated that coordinating
slow wave activity between layer-5 primary somatosensory cortex and secondary motor
cortex via synchronous optogenetic stimulation at 2 Hz enhances memory consolidation
of a newly learned non-declarative skill. Asynchronous stimulation of these two regions
(using opposite phases) reduced performance relative to the no-intervention controls
(Miyamoto et al., 2016).
These experiments (Maingret et al., 2016; Miyamoto et al., 2016; Latchoumane et al.,
2017) highlight the importance of both temporal and anatomical specificity of interventions
designed to boost the coupling between sleep oscillations across two brain regions.
Accordingly, a brief delay in stimulation timing was enough to abolish the memory
enhancement that is observed when locking stimulation accurately to HC SWRs (Maingret
et al., 2016).
How Can We Improve Causal Interventions in Humans Linking Sleep Oscillations to Learning
and Memory?
Over the last decade, several studies have gone beyond demonstrating the existence
of correlation between sleep oscillations (slow waves, spindles) and subsequent memory
recall (e.g., Gais et al., 2002; Huber et al., 2004, 2006; Mölle et al., 2009; Fogel
and Smith, 2011; Van Der Helm et al., 2011; Tamminen et al., 2013), to interventions
that link an experimentally-induced increase in the amplitude of a sleep oscillation
to human learning (Marshall et al., 2006; Ngo et al., 2013; Ladenbauer et al., 2017,
but also see Bueno-Lopez et al., 2019). A recent study demonstrated that causal interventions
affecting memory consolidation may also be applied locally. Unilateral olfactory stimulation
induced “local targeted memory reactivation” and elicited both behavioral and EEG
effects that were largely lateralized to one hemisphere (see preprint at - Bar et
al., 2019). Such lateralization seems more difficult to demonstrate in the auditory
modality (Simor et al., 2018), possibly because cortical auditory processing is less
lateralized compared to vision and olfaction (Schnupp et al., 2011).
One line of causal interventions during sleep employs a temporally tuned approach,
to perform “closed-loop” stimulation, phase-locked to endogenous sleep oscillations.
For example, auditory stimulation in phase with slow wave up-states (as measured with
scalp EEG) enhances slow wave activity and slow wave-spindle coupling, and improves
the consolidation of declarative memory (Ngo et al., 2013; Lafon et al., 2017; Ketz
et al., 2018; Goldi et al., 2019). Given that the timing of sleep oscillations differs
across cortical regions, choosing a specific EEG channel to trigger stimulation, phase-locks
the intervention to the timing of a specific cortical region. An elegant human study
that took this into consideration shows degradation of learning efficiency following
focal perturbation of slow wave activity over the motor cortex (Fattinger et al.,
2017). Importantly, the perturbation was ineffective when targeting temporo-parietal
cortex slow waves (Fattinger et al., 2017). Such an experimental approach draws our
attention to the role of local sleep oscillations in specific cortical areas for consolidation
of different types of memory tasks. The exact timing of intervention is critical for
enhancing memory consolidation, and changing the stimulation phase may abolish memory
effects completely (Ngo et al., 2013; Goldi et al., 2019).
Though impossible to directly compare, memory enhancement in humans appears to be
modest and less pronounced compared to memory enhancement following interventions
manipulating spindles and SWRs in rodents (Maingret et al., 2016; Latchoumane et al.,
2017). We suggest that the precise timing of the intervention is critical for memory
enhancement and may constitute an obstacle we need to overcome to obtain larger effects
in human subjects. At present, human interventions typically rely on scalp EEG summating
neuronal activity across wide regions, whereas animal studies track activity of specific
neural populations in deep brain areas.
When studying coherence of EEG sleep oscillations between different cortical sites
in humans, an important consideration is the tight and often underappreciated relation
between (i) the amplitude of a sleep oscillation (e.g., slow wave or sleep spindle)
as recorded with scalp EEG or intracranially, and (ii) its coherent occurrence across
neuronal ensembles. Put simply, high-amplitude oscillations often reflect high synchronization
between neuronal populations. Indeed we have shown, based on local iEEG recordings,
that the amplitude of each slow wave recorded on the scalp is tightly correlated with
the number of distant brain regions where this wave occurs near-simultaneously, such
that high-amplitude slow waves are global (Nir et al., 2011). In the case of sleep
spindles, high-amplitude events in scalp EEG likely reflect a precise coordination
among neurons in cortex, thalamus, and reticular thalamic nucleus (Nunez, 1995). This
means that many findings that link EEG slow wave or spindle amplitude/power in a given
region to learning and memory may in fact imply stronger coherence within relevant
neuronal circuits. Notwithstanding this, other factors also influence the amplitude
of EEG sleep oscillations, as asynchronous local generators can also produce an unexpectedly
large scalp signal (Von Ellenrieder et al., 2016). Further research is needed in order
to separate the contribution of high oscillatory power vs. high coherence between
specific areas to memory consolidation.
Future Outlooks
Technological advances should allow accurate mapping of the roles of specific spatially-circumscribed
cortical sleep events in the consolidation of long term memory in humans, and separate
them from other functions carried out by events that travel and encompass the whole
cortex. We expect that maturation of novel electrophysiology tools will improve both
spatial and temporal resolutions of monitoring human brain activity in real-time (Khodagholy
et al., 2017; Liu et al., 2018), thereby allowing accurate experimental interventions
in humans and improving their electrophysiological and cognitive effects. For such
advances to make an impact on basic scientific understanding and create genuine clinical
utility, it is imperative that theory is fine-tuned according to the available data,
and that we go beyond considering the sleeping brain as a uniform coherent entity.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to
the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial
or financial relationships that could be construed as a potential conflict of interest.
Related collections
Most cited references60
- Record: found
- Abstract: found
- Article: not found
Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration.
Giulio Tononi, Chiara Cirelli (2014)
- Record: found
- Abstract: found
- Article: not found
Auditory closed-loop stimulation of the sleep slow oscillation enhances memory.
Hong-Viet V. Ngo, Thomas Martinetz, Jan Born … (2013)
- Record: found
- Abstract: found
- Article: not found
Natural waking and sleep states: a view from inside neocortical neurons.
M Steriade, I Timofeev, F Grenier (2001)