- Record: found
- Abstract: found
- Article: found
Assessing Treatment Response of Glioblastoma to an HDAC Inhibitor Using Whole-Brain Spectroscopic MRI
Read this article at
Abstract
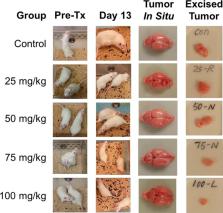
Histone deacetylases regulate a wide variety of cellular functions and have been implicated in redifferentiation of various tumors. Histone deacetylase inhibitors (HDACi) are potential pharmacologic agents to improve outcomes for patients with gliomas. We assessed the therapeutic efficacy of belinostat (PXD-101), an HDACi with blood–brain barrier permeability. Belinostat was first tested in an orthotopic rat glioma model to assess in vivo tumoricidal effect. Our results showed that belinostat was effective in reducing tumor volume in the orthotopic rat glioma model in a dose-dependent manner. We also tested the antidepression activity of belinostat in 2 animal models of depression and found it to be effective. Furthermore, we confirmed that myo-inositol levels improved by belinostat treatment in vitro. In a human pilot study, it was observed that belinostat in combination with chemoradiation may delay initial recurrence of disease. Excitingly, belinostat significantly improved depressive symptoms in patients with glioblastoma compared with control subjects. Finally, spectroscopic magnetic resonance imaging of 2 patient cases from this pilot study are presented to indicate how spectroscopic magnetic resonance imaging can be used to monitor metabolite response and assess treatment effect on whole brain. This study highlights the potential of belinostat to be a synergistic therapeutic agent in the treatment of gliomas.
Related collections
Most cited references17
- Record: found
- Abstract: found
- Article: not found
The Inventory of Depressive Symptomatology (IDS): psychometric properties.
- Record: found
- Abstract: found
- Article: not found
The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice.

- Record: found
- Abstract: found
- Article: found