- Record: found
- Abstract: found
- Article: found
Dendritic Cells Pulsed with Exosomes in Combination with PD-1 Antibody Increase the Efficacy of Sorafenib in Hepatocellular Carcinoma Model 1 2
Read this article at
Abstract
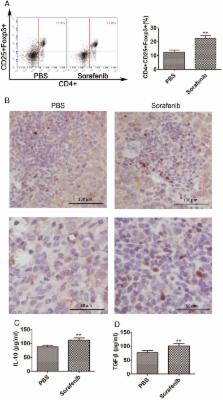
Advanced hepatocellular carcinoma (HCC) has limited therapeutic options. Immunotherapy is a promising treatment, while sorafenib is a first-line drug-based treatment for advanced HCC. However, the efficacy of sorafenib and immunotherapy in combination, have not been clearly evaluated. Sorafenib treatment has been shown to promote immunosuppression by increasing hypoxia in orthotopic HCC models. Here, we found that sorafenib treatment in mice with orthotopic HCC increased the expression of inhibitor programmed death-ligand 1 (PD-L1) and T-regulatory cells in tumor tissues. We pulsed dendritic cells with exosomes derived from tumor cells (DC-TEX) and found that the number of T-regulatory cells decreased and the number of CD8+T cells increased. However, combining DC-TEX and sorafenib did not prolong survival in these mice. Moreover, we found that the number of PD-1+CD8+T cells significantly increased after DC-TEX treatment. Therefore, we next added PD-1 antibody (PD-1 Ab) to the treatment regimen to block the PD-1/PD-L1 pathway, and found that the exhausted CD8+T cells were restored, without affecting the number of T-regulatory cells. Thus, our data suggest that the combination of DC-TEX and PD-1 Ab enhanced the efficacy of sorafenib, but treatment with either DC-TEX or PD-1 Ab alone, did not.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers.
- Record: found
- Abstract: found
- Article: not found
Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling.
- Record: found
- Abstract: found
- Article: not found