- Record: found
- Abstract: found
- Article: found
Elevated CXCL1 expression in breast cancer stroma predicts poor prognosis and is inversely associated with expression of TGF-β signaling proteins
Read this article at
Abstract
Background
CXCL1 is a chemotactic cytokine shown to regulate breast cancer progression and chemo-resistance. However, the prognostic significance of CXCL1 expression in breast cancer has not been fully characterized. Fibroblasts are important cellular components of the breast tumor microenvironment, and recent studies indicate that this cell type is a potential source of CXCL1 expression in breast tumors. The goal of this study was to further characterize the expression patterns of CXCL1 in breast cancer stroma, determine the prognostic significance of stromal CXCL1 expression, and identify factors affecting stromal CXCL1 expression.
Methods
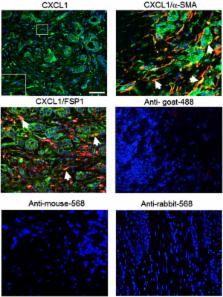
Stromal CXCL1 protein expression was analyzed in 54 normal and 83 breast carcinomas by immunohistochemistry staining. RNA expression of CXCL1 in breast cancer stroma was analyzed through data mining in http://www.Oncomine.org. The relationships between CXCL1 expression and prognostic factors were analyzed by univariate analysis. Co-immunofluorescence staining for CXCL1, α-Smooth Muscle Actin (α-SMA) and Fibroblast Specific Protein 1 (FSP1) expression was performed to analyze expression of CXCL1 in fibroblasts. By candidate profiling, the TGF-β signaling pathway was identified as a regulator of CXCL1 expression in fibroblasts. Expression of TGF-β and SMAD gene products were analyzed by immunohistochemistry and data mining analysis. The relationships between stromal CXCL1 and TGF-β signaling components were analyzed by univariate analysis. Carcinoma associated fibroblasts isolated from MMTV-PyVmT mammary tumors were treated with recombinant TGF-β and analyzed for CXCL1 promoter activity by luciferase assay, and protein secretion by ELISA.
Results
Elevated CXCL1 expression in breast cancer stroma correlated with tumor grade, disease recurrence and decreased patient survival. By co-immunofluorescence staining, CXCL1 expression overlapped with expression of α-SMA and FSP1 proteins. Expression of stromal CXCL1 protein expression inversely correlated with expression of TGF-β signaling components. Treatment of fibroblasts with TGF-β suppressed CXCL1 secretion and promoter activity.
Conclusions
Increased CXCL1 expression in breast cancer stroma correlates with poor patient prognosis. Furthermore, CXCL1 expression is localized to α-SMA and FSP1 positive fibroblasts, and is negatively regulated by TGF-β signaling. These studies indicate that decreased TGF-β signaling in carcinoma associated fibroblasts enhances CXCL1 expression in fibroblasts, which could contribute to breast cancer progression.
Related collections
Most cited references75
- Record: found
- Abstract: found
- Article: found
Stromal gene expression predicts clinical outcome in breast cancer.
- Record: found
- Abstract: found
- Article: not found
Identification of fibroblast heterogeneity in the tumor microenvironment.
- Record: found
- Abstract: found
- Article: not found