- Record: found
- Abstract: found
- Article: found
Comparative effectiveness of first-line palbociclib plus letrozole versus letrozole alone for HR+/HER2− metastatic breast cancer in US real-world clinical practice
Read this article at
Abstract
Background
Findings from randomized clinical trials may have limited generalizability to patients treated in routine clinical practice. This study examined the effectiveness of first-line palbociclib plus letrozole versus letrozole alone on survival outcomes in patients with hormone receptor–positive (HR+)/human epidermal growth factor receptor–negative (HER2−) metastatic breast cancer (MBC) treated in routine clinical practice in the USA.
Patients and methods
This was a retrospective observational analysis of electronic health records within the Flatiron Health Analytic Database. A total of 1430 patients with ≥ 3 months of follow-up received palbociclib plus letrozole or letrozole alone in the first-line setting between February 3, 2015, and February 28, 2019. Stabilized inverse probability treatment weighting (sIPTW) was used to balance baseline demographic and clinical characteristics. Real-world progression-free survival (rwPFS) and overall survival (OS) were analyzed.
Results
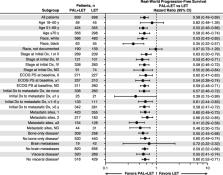
After sIPTW adjustment, median follow-up was 24.2 months (interquartile range [IQR], 14.2–34.9) in the palbociclib group and 23.3 months (IQR, 12.7–34.3) in those taking letrozole alone. Palbociclib combination treatment was associated with significantly longer median rwPFS compared to letrozole alone (20.0 vs 11.9 months; hazard ratio [HR], 0.58; 95% CI, 0.49–0.69; P < 0.0001). Median OS was not reached in the palbociclib group and was 43.1 months with letrozole alone (HR, 0.66; 95% CI, 0.53–0.82; P = 0.0002). The 2-year OS rate was 78.3% in the palbociclib group and 68.0% with letrozole alone. A propensity score matching analysis showed similar results.
Related collections
Most cited references23
- Record: found
- Abstract: not found
- Article: not found
The central role of the propensity score in observational studies for causal effects

- Record: found
- Abstract: found
- Article: not found