- Record: found
- Abstract: found
- Article: found
Impaired Cerebral Autoregulation Is Associated with Brain Atrophy and Worse Functional Status in Chronic Ischemic Stroke
Read this article at
Abstract
Dynamic cerebral autoregulation (dCA) is impaired following stroke. However, the relationship between dCA, brain atrophy, and functional outcomes following stroke remains unclear. In this study, we aimed to determine whether impairment of dCA is associated with atrophy in specific regions or globally, thereby affecting daily functions in stroke patients.
We performed a retrospective analysis of 33 subjects with chronic infarctions in the middle cerebral artery territory, and 109 age-matched non-stroke subjects. dCA was assessed via the phase relationship between arterial blood pressure and cerebral blood flow velocity. Brain tissue volumes were quantified from MRI. Functional status was assessed by gait speed, instrumental activities of daily living (IADL), modified Rankin Scale, and NIH Stroke Score.
Compared to the non-stroke group, stroke subjects showed degraded dCA bilaterally,
and showed gray matter atrophy in the frontal, parietal and temporal lobes ipsilateral
to infarct. In stroke subjects, better dCA was associated with less temporal lobe
gray matter atrophy on the infracted side (
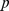 = 0.029), faster gait speed (
= 0.029), faster gait speed (
 = 0.018) and lower IADL score (
= 0.018) and lower IADL score (
 0.002). Our results indicate that better dynamic cerebral perfusion regulation is
associated with less atrophy and better long-term functional status in older adults
with chronic ischemic infarctions.
0.002). Our results indicate that better dynamic cerebral perfusion regulation is
associated with less atrophy and better long-term functional status in older adults
with chronic ischemic infarctions.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Cerebral autoregulation.
- Record: found
- Abstract: found
- Article: not found
Telmisartan to prevent recurrent stroke and cardiovascular events.
- Record: found
- Abstract: found
- Article: not found