- Record: found
- Abstract: found
- Article: found
Emerging Roles in the Biogenesis of Cytochrome c Oxidase for Members of the Mitochondrial Carrier Family
Read this article at
Abstract
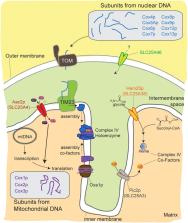
The mitochondrial carrier family (MCF) is a group of transport proteins that are mostly localized to the inner mitochondrial membrane where they facilitate the movement of various solutes across the membrane. Although these carriers represent potential targets for therapeutic application and are repeatedly associated with human disease, research on the MCF has not progressed commensurate to their physiologic and pathophysiologic importance. Many of the 53 MCF members in humans are orphans and lack known transport substrates. Even for the relatively well-studied members of this family, such as the ADP/ATP carrier and the uncoupling protein, there exist fundamental gaps in our understanding of their biological roles including a clear rationale for the existence of multiple isoforms. Here, we briefly review this important family of mitochondrial carriers, provide a few salient examples of their diverse metabolic roles and disease associations, and then focus on an emerging link between several distinct MCF members, including the ADP/ATP carrier, and cytochrome c oxidase biogenesis. As the ADP/ATP carrier is regarded as the paradigm of the entire MCF, its newly established role in regulating translation of the mitochondrial genome highlights that we still have a lot to learn about these metabolite transporters.
Related collections
Most cited references245
- Record: found
- Abstract: found
- Article: not found
The SLC2 (GLUT) family of membrane transporters.
- Record: found
- Abstract: found
- Article: not found
Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form.
- Record: found
- Abstract: found
- Article: not found