- Record: found
- Abstract: found
- Article: not found
Differential Epidermal Growth Factor Receptor Signaling Regulates Anchorage-Independent Growth by Modulation of the PI3K/AKT Pathway
Abstract
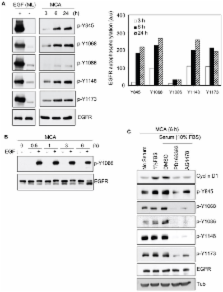
Tumor cells are capable of surviving loss of nutrients and anchorage in hostile microenvironments. Under these conditions adapting to specific signaling pathways may shift the balance between growth and cellular dormancy. Here we report a mechanism by which EGFR differentially modulates the PI3K/AKT pathway in cellular stress conditions. When carcinoma cells were cultured as multicellular aggregates (MCA), cyclin D1 was induced through a serum-dependent EGFR activating pathway, triggering cell proliferation. The expression of cyclin D1 required both EGFR-mediated ERK and AKT activation. In serum-starved MCAs, EGFR activation was associated with active ERK1/2 but not AKT and failed to induce cyclin D1. Analysis revealed that, under serum-starved conditions, EGFR-Y1086 residue was poorly autophosphorylated and this correlated with failure to phosphorylate Gab1. Accordingly, the EGFR activation failed to induce EGFR/PI3K complex formation or AKT activation, preventing cyclin D1 induction. Furthermore, we show that in serum-starved MCA, expression of constitutively active AKT re-established cyclin D1 expression and induced proliferation in an EGFR-dependent manner. Thus, modulation of the PI3K/AKT pathway by context-dependent EGFR signaling may regulate tumor cell growth and dormancy.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
