- Record: found
- Abstract: found
- Article: found
Interventional therapy for human breast cancer in nude mice with 131I gelatin microspheres ( 131I-GMSs) following intratumoral injection
Read this article at
Abstract
Introduction
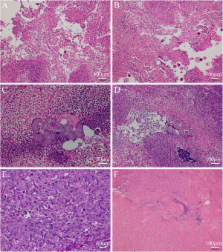
The aim of this study was to investigate the effects of 131I gelatin microspheres ( 131I-GMS) on human breast cancer cells (MCF-7) in nude mice and the biodistribution of 131I-GMSs following intratumoral injections.
Methods
A total of 20 tumor-bearing mice were divided into a treatment group and control group and received intratumoral injections of 2.5 mci 131I-GMSs and nonradioactive GMSs, respectively. Tumor size was measured once per week. Another 16 mice received intratumoral injections of 0.4 mci 131I-GMSs and were subjected to single photon emission computed tomography (SPECT) scans and tissue radioactivity concentration measurements on day 1, 4, 8 and 16 postinjection. The 20 tumor-bearing mice received intratumoral injections of 0.4 mci [ 131I] sodium iodide solution and were subjected to SPECT scans and intratumoral radioactivity measurements at 1, 6, 24, 48 and 72 h postinjection. The tumors were collected for histological examination.
Results
The average tumor volume in the 131I-GMSs group on post-treatment day 21 decreased to 86.82 ± 63.6%, while it increased to 893.37 ± 158.12% in the control group ( P < 0.01 vs. the 131I-GMSs group). 131I-GMSs provided much higher intratumoral retention of radioactivity, resulting in 19.93 ± 5.24% of the injected radioactivity after 16 days, whereas the control group retained only 1.83 ± 0.46% of the injected radioactivity within the tumors at 1 h postinjection.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
Twenty-Year Follow-up of a Randomized Trial Comparing Total Mastectomy, Lumpectomy, and Lumpectomy plus Irradiation for the Treatment of Invasive Breast Cancer
- Record: found
- Abstract: found
- Article: not found
Gelatin as a delivery vehicle for the controlled release of bioactive molecules.
- Record: found
- Abstract: found
- Article: not found