- Record: found
- Abstract: found
- Article: found
Abortigenic but Not Neurotropic Equine Herpes Virus 1 Modulates the Interferon Antiviral Defense
Read this article at
Abstract
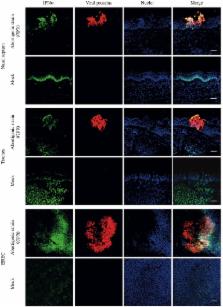
Equine herpesvirus 1 (EHV1) is considered as a major pathogen of Equidae, causing symptoms from mild respiratory disease to late-term abortion and neurological disorders. Different EHV1 strains circulating in the field have been characterized to be of abortigenic or neurovirulent phenotype. Both variants replicate in a plaque-wise manner in the epithelium of the upper respiratory tract (URT), where the abortigenic strains induce more prominent viral plaques, compared to the neurovirulent strains. Considering the differences in replication at the URT, we hypothesized that abortigenic strains may show an increased ability to modulate the type I IFN secretion/signaling pathway, compared to strains that display the neurovirulent phenotype. Here, we analyze IFN levels induced by abortigenic and neurovirulent EHV1 using primary respiratory epithelial cells (EREC) and respiratory mucosa ex vivo explants. Similar levels of IFNα (~70 U/ml) were detected in explants inoculated with both types of EHV1 strains from 48 to 72 hpi. Second, EREC and mucosa explants were treated with recombinant equine IFNα (rEqIFNα) or Ruxolitinib (Rux), an IFN signaling inhibitor, prior to and during inoculation with abortigenic or neurovirulent EHV1. Replication of both EHV1 variants was suppressed by rEqIFNα. Further, addition of Rux increased replication in a concentration-dependent manner, indicating an IFN-susceptibility for both variants. However, in two out of three horses, at a physiological concentration of 100 U/ml of rEqIFNα, an increase in abortigenic EHV1 replication was observed compared to 10 U/ml of rEqIFNα, which was not observed for the neurovirulent strains. Moreover, in the presence of Rux, the plaque size of the abortigenic variants remained unaltered, whereas the typically smaller viral plaques induced by the neurovirulent variants became larger. Overall, our results demonstrate the importance of IFNα in the control of EHV1 replication in the URT for both abortigenic and neurovirulent variants. In addition, our findings support the speculation that abortigenic variants of EHV1 may have developed anti-IFN mechanisms that appear to be absent or less pronounced in neurovirulent EHV1 strains.
Related collections
Most cited references67
- Record: found
- Abstract: found
- Article: not found
The airway epithelium: soldier in the fight against respiratory viruses.
- Record: found
- Abstract: found
- Article: not found
Interferons and viruses: an evolutionary arms race of molecular interactions.
- Record: found
- Abstract: found
- Article: not found