- Record: found
- Abstract: found
- Article: not found
GILT is a critical host factor for Listeria monocytogenes infection
Abstract
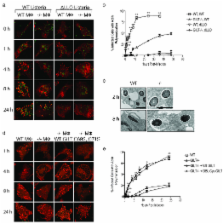
Listeria monocytogenes is a gram positive, intracellular, food-borne pathogen that can cause severe illness in humans and animals. Upon infection, it is actively phagocytosed by macrophages 1. It then escapes from the phagosome, replicates in the cytosol, and subsequently spreads from cell to cell by a non-lytic mechanism driven by actin polymerization 2. Penetration of the phagosomal membrane is initiated by the secreted hemolysin listeriolysin O (LLO), which is essential for vacuolar escape in vitro and for virulence in animal models of infection 3. Reduction is required to activate the lytic activity of LLO in vitro 4– 6, and we show here that reduction by the enzyme Gamma-interferon Inducible Lysosomal Thiolreductase (GILT) is responsible for the activation of LLO in vivo. GILT is a soluble thiol reductase expressed constitutively within the lysosomes of antigen presenting cells 7, 8, and it accumulates in macrophage phagosomes as they mature into phagolysosomes 9. The enzyme is delivered by a mannose-6-phosphate receptor-dependent mechanism to the endocytic pathway, where N- and C-terminal pro-peptides are cleaved to generate a 30 kDa mature enzyme 7, 8, 10. The active site of GILT contains two cysteine residues in a CXXC motif that catalyzes the reduction of disulfide bonds 7, 8. Mice lacking GILT are deficient in generating MHC class II-restricted CD4 + T cell responses to protein antigens that contain disulfide bonds 11, 12. Here we show that these mice are resistant to L. monocytogenes infection. Replication of the organism in GILT-negative macrophages, or macrophages expressing an enzymatically inactive GILT mutant, is impaired because of delayed escape from the phagosome. GILT activates LLO within the phagosome by the classical thiol reductase mechanism shared by members of the thioredoxin family. In addition, purified GILT activates recombinant LLO, facilitating membrane permeabilization and red blood cell lysis. The data show GILT is a critical host factor that facilitates L. monocytogenes infection.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes
- Record: found
- Abstract: found
- Article: not found
The Phagosome Proteome
- Record: found
- Abstract: found
- Article: not found
