- Record: found
- Abstract: found
- Article: found
Interleukin (IL) 16: a candidate urinary biomarker for proliferative lupus nephritis
Read this article at
Abstract
Objective
Lupus nephritis (LN) is a severe manifestation of systemic lupus erythematosus (SLE). The pathogenesis is incompletely understood and diagnostic biomarkers are scarce. We investigated interleukin (IL) 16 as a potential biomarker for LN in a well-characterised cohort of patients with SLE.
Methods
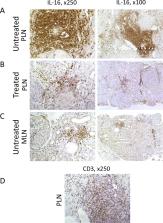
We measured urinary (u-) and plasma (p-) levels of IL-16 in predefined patient groups using ELISA: LN (n=84), active non-renal SLE (n=63), inactive non-renal SLE (n=73) and matched population controls (n=48). The LN group included patients with recent biopsy-confirmed proliferative (PLN, n=47), mesangioproliferative (MES, n=11) and membranous (MLN, n=26) LN. Renal expression of IL-16 was investigated by immunohistochemistry. Associations between IL-16 measurements and clinical parameters and the diagnostic value for LN were explored.
Results
p-IL-16 was detected in all investigated cases and high p-IL-16 levels were observed in patients with active SLE. u-IL-16 was detected (dt-u-IL-16) in 47.6% of patients with LN, while only up to 17.8% had dt-u-IL-16 in other groups. In the LN group, 68% of patients with PLN had dt-u-IL-16, while the proportions in the MLN and MES groups were lower (11.5% and 45.5%, respectively). The highest u-IL-16 levels were detected in the PLN group. In the regression model, u-IL-16 levels differentiated PLN from other LN patient subgroups (area under the curve 0.775–0.896, p<0.0001). dt-u-IL-16 had superior specificity but slightly lower sensitivity than elevated anti-double-stranded DNA and low complement C3 or C4 in diagnosing PLN. A high proportion of LN kidney infiltrating cells expressed IL-16.
Conclusions
We demonstrate that detectable u-IL-16 can differentiate patients with PLN from those with less severe LN subtypes and active non-renal SLE. Our findings suggest that u-IL-16 could be used as a screening tool at suspicion of severe LN. Furthermore, the high IL-16 levels in plasma, urine and kidney tissue imply that IL-16 could be explored as a therapeutic target in SLE.
Related collections
Most cited references34

- Record: found
- Abstract: found
- Article: found
QuPath: Open source software for digital pathology image analysis
- Record: found
- Abstract: found
- Article: not found
Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus.
- Record: found
- Abstract: found
- Article: not found