- Record: found
- Abstract: found
- Article: found
Restoration of miR-29b exerts anti-cancer effects on glioblastoma
Read this article at
Abstract
Background
Glioblastoma multiforme (GBM) is known as one of the most fatal forms of cancer. MicroRNAs have been widely implicated in the regulation of mammalian development and pathogenesis. The brain-enriched miR-29 subfamilies are known to be exclusively expressed in the developing brain, and they are aberrantly down-regulated in GBM. This study aims to elucidate the role of miR-29b in GBM development and the feasibility of therapeutic targeting using conjugated nanoparticles.
Methods
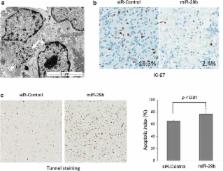
After confirmation of miR-29b expression levels in GBM tissues by analysis of open source data, the anticancer effect of miR-29b was tested by the introduction of syn-hsa-miR-29b-3p in the A172 GBM cell line. In vitro studies of cell viability and apoptosis and ex vivo study using GBM tissue slice cultures from 3 patients and nanoparticle delivery of miR-29b were performed.
Results
We discovered an increase in apoptotic cell populations with the introduction of miR-29b in the GBM cell line. An established human-derived GBM tissue slice culture system confirmed the anticancer effect of miR-29b-conjugated nanoparticles. Using PCR array, we found that exogenous miR-29b inhibits the expression of COL1A2, COL3A1, COL4A1, ELN, ITGA11, MMP24, and SPARC, which mediates an anticancer effect.
Related collections
Most cited references12
- Record: found
- Abstract: found
- Article: not found
MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells.
- Record: found
- Abstract: found
- Article: not found
A hexanucleotide element directs microRNA nuclear import.
- Record: found
- Abstract: found
- Article: not found