- Record: found
- Abstract: found
- Article: found
Cyp19a1 (Aromatase) Expression in the Xenopus Brain at Different Developmental Stages
Read this article at
Abstract
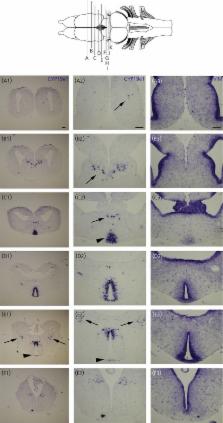
Cytochrome P450 aromatase (P450arom; aromatase) is a microsomal enzyme involved in the production of endogeneous sex steroids by converting testosterone into oestradiol. Aromatase is the product of the cyp19a1 gene and plays a crucial role in the sexual differentiation of the brain and in the regulation of reproductive functions. In the brain of mammals and birds, expression of cyp19a1 has been demonstrated in neuronal populations of the telencephalon and diencephalon. By contrast, a wealth of evidence established that, in teleost fishes, aromatase expression in the brain is restricted to radial glial cells. The present study investigated the precise neuroanatomical distribution of cyp19a1 mRNA during brain development in Xenopus laevis (late embryonic to juvenile stages). For this purpose, we used in situ hybridisation alone or combined with the detection of a proliferative (proliferating cell nuclear antigen), glial (brain lipid binding protein, Vimentin) or neuronal (acetylated tubulin; HuC/D; NeuroβTubulin) markers. We provide evidence that cyp19a1 expression in the brain is initiated from the very early larval stage and remains strongly detected until the juvenile and adult stages. At all stages analysed, we found the highest expression of cyp19a1 in the preoptic area and the hypothalamus compared to the rest of the brain. In these two brain regions, cyp19a1-positive cells were never detected in the ventricular layers. Indeed, no co-labelling could be observed with radial glial (brain lipid binding protein, Vimentin) or dividing progenitors (proliferating cell nuclear antigen) markers. By contrast, cyp19a1-positive cells perfectly matched with the distribution of post-mitotic neurones as shown by the use of specific markers (HuC/D, acetylated tubulin and NeuroβTubulin). These data suggest that, similar to that found in other tetrapods, aromatase in the brain of amphibians is found in post-mitotic neurones and not in radial glia as reported in teleosts.
Related collections
Most cited references79
- Record: found
- Abstract: found
- Article: not found
Aromatase--a brief overview.
- Record: found
- Abstract: found
- Article: not found
Estrogen masculinizes neural pathways and sex-specific behaviors.
- Record: found
- Abstract: found
- Article: not found